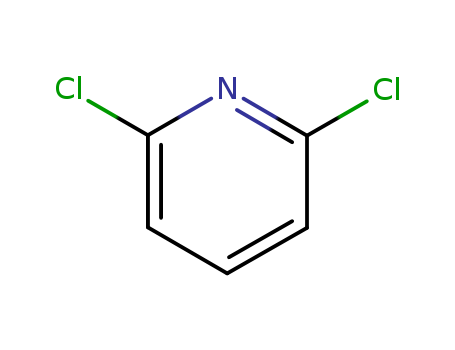
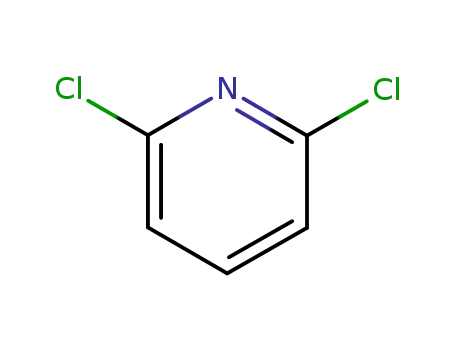
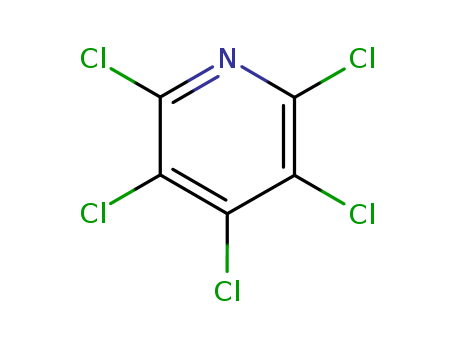
Buy High Grade Chinese Manufacturer Supply 2,6-Dichloropyridine 2402-78-0 with Low Price
- Molecular Formula:C5H3Cl2N
- Molecular Weight:147.992
- Appearance/Colour:White to light yellow solid
- Vapor Pressure:0.349mmHg at 25°C
- Melting Point:83-86 °C(lit.)
- Refractive Index:1.553
- Boiling Point:206 °C at 760 mmHg
- PKA:-3.02±0.10(Predicted)
- Flash Point:97.5 °C
- PSA:12.89000
- Density:1.388 g/cm3
- LogP:2.38840
2,6-Dichloropyridine(Cas 2402-78-0) Usage
|
Purification Methods
|
It crystallises from EtOH. [Beilstein 20/5 V 416.]
|
|
Consumer Uses
|
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment.
|
InChI:InChI=1/C5H3Cl2N/c6-4-2-1-3-5(7)8-4/h1-3H
2402-78-0 Relevant articles
-
McKendry,Muelder
, p. 87,94 (1978)
-
Highly Chemoselective Deoxygenation of N-Heterocyclic N-Oxides Using Hantzsch Esters as Mild Reducing Agents
An, Ju Hyeon,Kim, Kyu Dong,Lee, Jun Hee
supporting information, p. 2876 - 2894 (2021/02/01)
Herein, we disclose a highly chemoselect...
Production method of 2, 6-dichloropyridine
-
Paragraph 0027-0060, (2020/05/01)
The invention discloses a production met...
Catalyst-Free N-Deoxygenation by Photoexcitation of Hantzsch Ester
Cardinale, Luana,Jacobi Von Wangelin, Axel,Konev, Mikhail O.
supporting information, (2020/02/15)
A mild and operationally simple protocol...
Method for preparing 2,6-dichloropyridine by pyridine liquid-phase photochlorination
-
Paragraph 0016-0027; 0028; 0029; 0031, (2019/01/23)
The invention relates to a method for pr...
2402-78-0 Process route
-
- 1073-67-2,24991-47-7
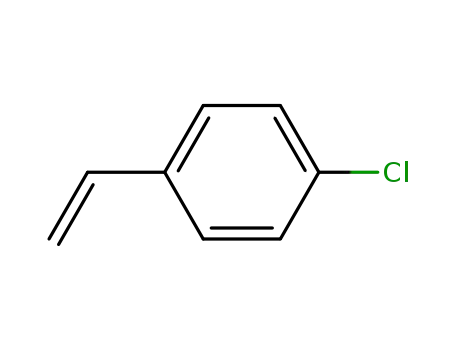
4-vinylbenzyl chloride
-
- 2587-00-0
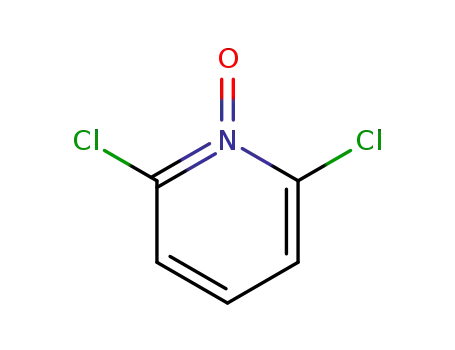
2,6-dichloropyridine N-oxide
-
- 2402-78-0
2,6-dichloropyridine
-
- 2788-86-5,53649-47-1,97466-49-4,21019-51-2
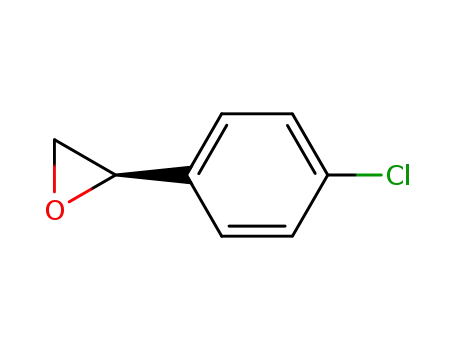
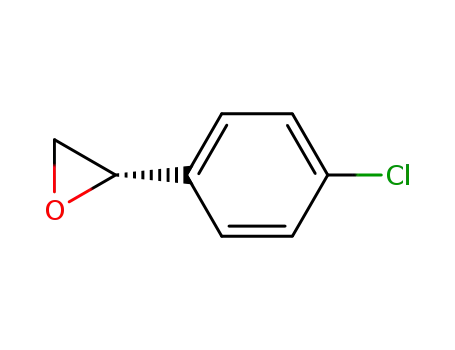
(R)-2-(4-chlorophenyl)oxirane
-
- 2788-86-5,21019-51-2,53649-47-1,97466-49-4
(2S)-2-(4-chlorophenyl)oxirane
Conditions
| Conditions |
Yield |
|
chiral ruthenium porphyrin complex catalyst; In toluene; at -10 ℃; for 48h; Title compound not separated from byproducts.;
|
|
-
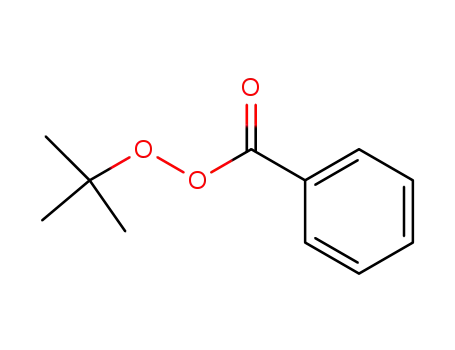
- 614-45-9
tert-Butyl peroxybenzoate
-
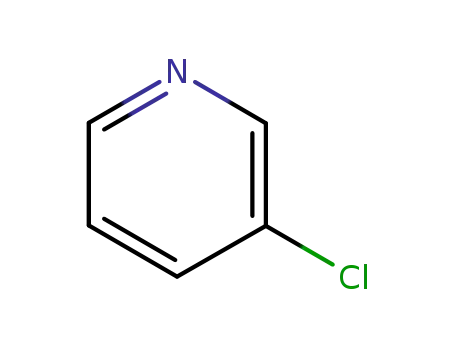
- 626-60-8
3-Chloropyridine
-
- 109-09-1
2-chloropyridine
-
- 626-61-9
4-Chloropyridine
-
- 2402-78-0
2,6-dichloropyridine
Conditions
| Conditions |
Yield |
|
pyridine; With chlorine; di-tert-butyl peroxide; In tetrachloromethane; water; at 231 - 244 ℃; for 0.00361111 - 0.00722222h;
tert-Butyl peroxybenzoate; Product distribution / selectivity;
|
35% |
2402-78-0 Upstream products
2402-78-0 Downstream products
-
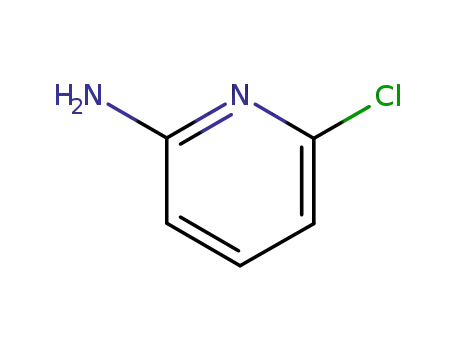
45644-21-1
6-chloropyridin-2-amine
-
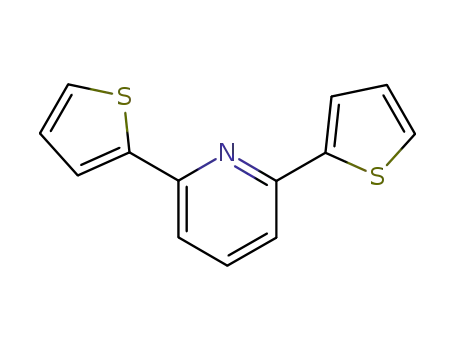
35299-71-9
2,6-bis(thiophen-2-yl)pyridine
-
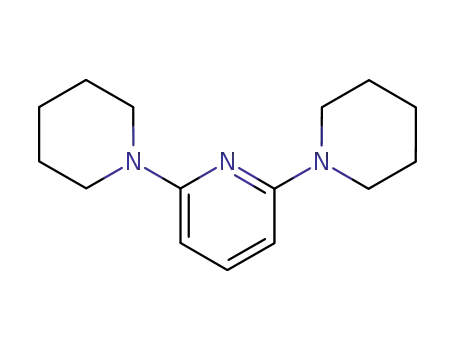
3980-49-2
2,6-di(piperidine-1-yl)pyridine
-
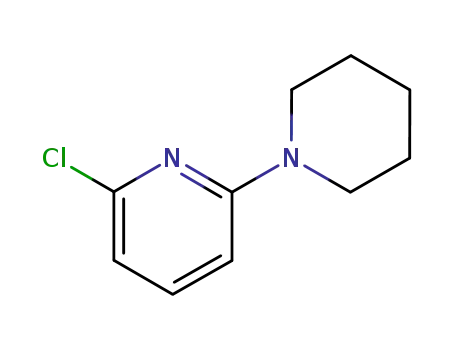
19946-28-2
2-chloro-6-(1-piperidyl)pyridine
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego