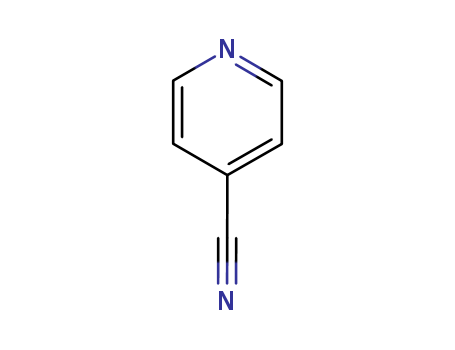
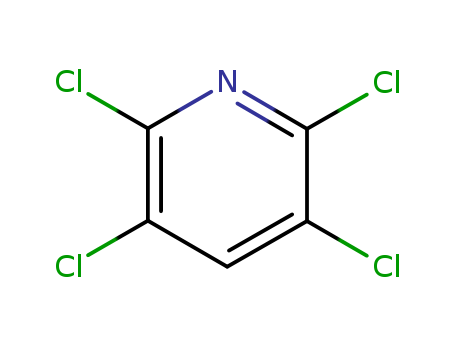
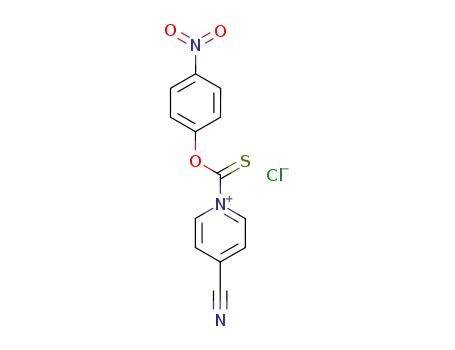
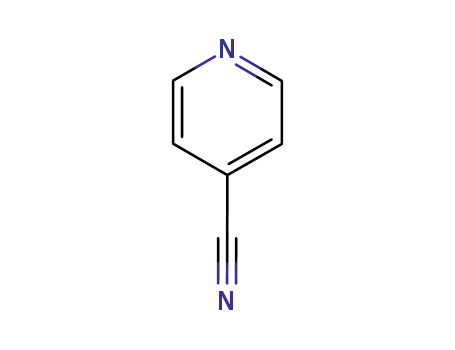
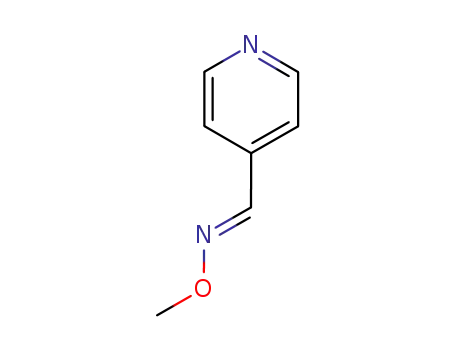
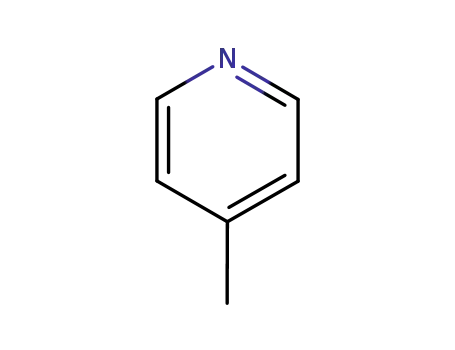
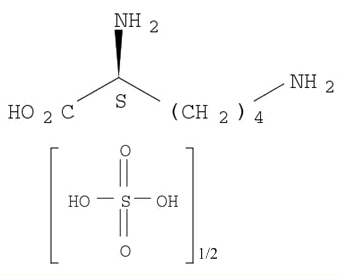
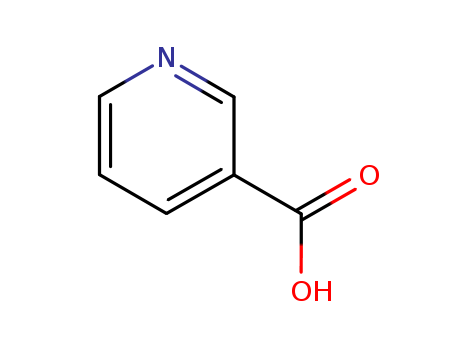
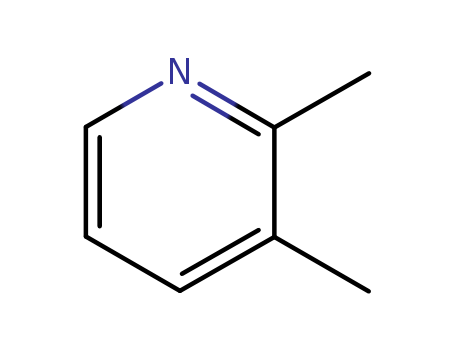
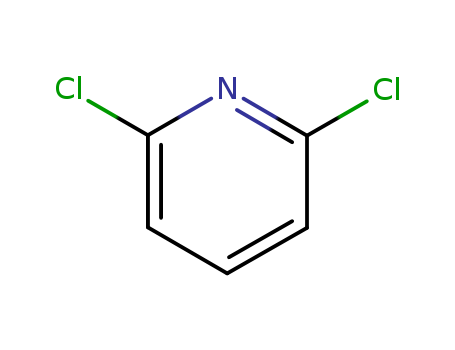
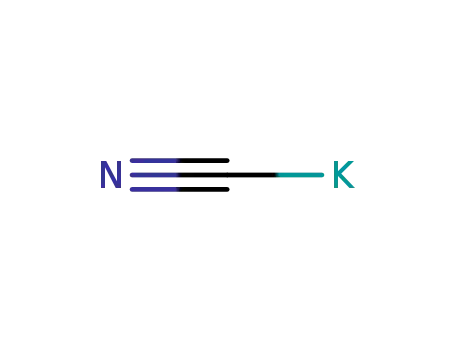An efficient new procedure for the one-pot conversion of aldehydes into the corresponding nitriles
Zhu, Jia-Liang,Lee, Fa-Yen,Wu, Jen-Dar,Kuo, Chun-Wei,Shia, Kak-Shan
, p. 1317 - 1319 (2007)
A new and efficient procedure for the on...
Synthesis of [5-(1H-1,2,3-triazol-4-yl)-1,3,4-oxadiazol-2-yl]pyridines
Pokhodylo,Shiika,Matiichuk,Obushak
, p. 417 - 421 (2010)
2-, 3-, and 4-[5-(1-Aryl-5-R-1H-1,2,3-tr...
Eine effiziente Synthese von 4-Cyanopyridin
Schantl, Joachim,Gstach, Hubert
, p. 694 - 695 (1980)
-
A simple and convenient method for the synthesis of nitriles by oxidation of primary amines with NaOCl in ethanol
Yamazaki, Shigekazu
, p. 3559 - 3564 (1997)
The oxidation of aliphatic primary amine...
An efficient one-pot synthesis of nitriles from carboxylic acids without solvent under microwave irradiation
Juncai, Feng,Bin, Liu,Yang, Lhi,Changchuan, Li
, p. 4545 - 4548 (1996)
Nitriles were prepared from alkyl and ar...
Deoxygenation of pyridine N-oxides with dimethylthiocarbamoyl chloride
Ponaras, Anthony A.,Zaim, Oemer
, p. 487 - 489 (2007)
(Chemical Equation Presented) Treatment ...
A mild deoxygenation of heteroaromatic N-oxides by formamidinesulfinic acid
Balicki, Roman,Chmielowiec, Urszula
, p. 1105 - 1107 (2000)
Various heteroaromatic N-oxides were eff...
Pentacyanoferrates(II): Solvatochromism and Reactivity in Micelles and in Reversed Micelles
Burgess, John,Patei, Marttand S.
, p. 783 - 788 (1993)
Rate constants for dissociation of pyrid...
Efficient and chemoselective deoxygenation of amine N-oxides using polymethylhydrosiloxane
Chandrasekhar,Reddy, Ch. Raji,Rao, R. Jagadeeshwar,Rao, J. Madhusudana
, p. 349 - 351 (2002)
Deoxygenation of aromatic and aliphatic ...
Electronic Effects on the Menschutkin Reaction. A Complete Kinetic and Thermodynamic Dissection of Alkyl Transfer to 3- and 4-Substituted Pyridines
Arnett, Edward M.,Reich, Ronald
, p. 5892 - 5902 (1980)
The relationship between kinetic and the...
Boryl Radicals-Triggered Selective C-H Functionalization for the Synthesis of Diverse Phenanthridine Derivatives
Guo, Ao,Han, Jia-Bin,Tang, Xiang-Ying
, p. 2351 - 2355 (2018)
A boryl radical-triggered C-H functional...
A NEW METHOD FOR DEOXYGENATION OF HETEROAROMATIC N-OXIDES WITH CHLOROTRIMETHYLSILANE/SODIUM IODIDE/ZINC
Morita, Tsuyoshi,Kuroda, Koji,Okamoto, Yoshiki,Sakurai, Hiroshi
, p. 921 - 924 (1981)
Heteroaromatic N-oxides are easily deoxy...
A Novel and Efficient Deoxygenation of Hetero Cyclic N-Oxides Using ZrCl4/NaBH4
Chary, K. Purushothama,Mohan, G. Hari,Iyengar, D. S.
, p. 1339 - 1340 (1999)
A practical and novel reagent system ZrC...
Outer-sphere and inner-sphere processes in organic chemistry. Reaction of trifluoromethyl bromide with electrochemically generated aromatic anion radicals and sulfur dioxide anion radicals
Andrieux,Gelis,Saveant
, p. 786 - 791 (1990)
The reduction of CF3Br by electrochemica...
Highly efficient gold nanoparticle catalyzed deoxygenation of amides, sulfoxides, and pyridine N-oxides
Mikami, Yusuke,Noujima, Akifumi,Mitsudome, Takato,Mizugaki, Tomoo,Jitsukawa, Koichiro,Kaneda, Kiyotomi
, p. 1768 - 1772 (2011)
Selective deoxygenation is one of the mo...
Efficient transformation of aldoximes to nitriles using 2-chloro-1-methylpyridinium iodide under mild conditions
Lee, Kieseung,Han, Sang-Bae,Yoo, Eun-Mi,Chung, Soon-Ryang,Oh, Haibum,Hong, Sungwan
, p. 1775 - 1782 (2004)
Various (aliphatic, aromatic, and hetero...
Efficient catalytic conversion of pyridine N-oxides to pyridine with an oxorhenium(V) catalyst
Wang, Ying,Espenson, James H.
, p. 3525 - 3526 (2000)
(Equation Presented) The compound CH3Re(...
Catalytic deoxygenation of pyridine N-oxides with N-fused porphyrin rhenium complexes
Toganoh, Motoki,Fujino, Keitaro,Ikeda, Shinya,Furuta, Hiroyuki
, p. 1488 - 1491 (2008)
Deoxygenation reactions of pyridine N-ox...
Efficient Deoxygenation of Heteroaromatic N-Oxides with Ammonium Formate as a Catalytic Hydrogen Transfer Agent
Balicki, Roman
, p. 645 - 646 (1989)
Ammonium formate catalytic transfer hydr...
-
Barlin,Brown
, p. 2473 (1967)
-
Catalyst-Free N-Deoxygenation by Photoexcitation of Hantzsch Ester
Cardinale, Luana,Jacobi Von Wangelin, Axel,Konev, Mikhail O.
, (2020)
A mild and operationally simple protocol...
Reaction of N-aminopyridinium salt with cyanide ion.
Okamoto,Hirobe,Tamai
, p. 1089 - 1090 (1963)
-
Facile and Efficient Deoxygenation of Amine-N-oxides with Gallium in Water
Han, Jung Hwa,Choi, Kyung Il,Kim, Joong Hyup,Yoo, Woo Byung
, p. 3197 - 3202 (2004)
A facile and efficient procedure for the...
Reactivity of Heterocyclic Nitrogen Donors in Systems containing the Tetrachloroaurate(III) Anion
Canovese, Luciano,Cattalini, Lucio,Tomaselli, Michele,Tobe, Martin L.
, p. 307 - 314 (1991)
A series of gold(III) complexes of the t...
Chemoselective hydrogenation of nitroarenes and deoxygenation of pyridine N-oxides with H2 catalyzed by MoO2Cl2
Reis, Patrícia M.,Royo, Beatriz
, p. 949 - 952 (2009)
A chemoselective and highly efficient hy...
Formation and Reaction of Trichloro Complexes of Bivalent Transition Metals in 1,2-Dichloroethane
Satoh, Keiichi,Suzuki, Toshio,Sawada, Kiyoshi
, p. 591 - 594 (1988)
Trichloro complexes of some bivalent tra...
Mild and efficient deoxygenation of amine-N-oxides with MoCl5/NaI system
Yoo, Byung Woo,Park, Min Chol
, p. 1646 - 1650 (2008)
The MoCl5/NaI system was found to be a n...
An Efficient Deoxgenation of Heteroaromatic N-Oxides Using Zinc Dust/Ammonium Formate Reagent System
Balicki, Roman,Cybulski, Marcin,Maciejewski, Grzegorz
, p. 4137 - 4141 (2003)
Heteroaromatic N-oxides were readily and...
An overview on the progress and development on the palladium catalyzed direct cyanation
Heydari, Somayyeh,Habibi, Davood,Reza Faraji, Ali,keypour, Hassan,Mahmoudabadi, Masoumeh
, (2021)
Generation of the positive CN ion and th...
Stability studies of bis(pyridiniumaldoxime) reactivators of organophosphate-inhibited acetylcholinesterase
Lin,Klayman
, p. 797 - 799 (1986)
Relative stability studies of three orga...
Facile deoxygenation of amine-N-oxides with CoCl2·6H 2O-indium system
Jung, Hwa Han,Kyung, Il Choi,Joong, Hyup Kim,Cheol, Min Yoon,Byung, Woo Yoo
, p. 415 - 419 (2006)
CoCl2·6H2O/In system was found to be a n...
Selective and efficient deoxygenation of amine-n-oxides with CeCl 3 7H2O/zinc system
Yoo, Byung Woo,Jung, Ha Il,Kim, Se Heon,Ahn, Young Sun,Choi, Ji Yong
, p. 359 - 360 (2013)
-
Interconversion of MeReO(dithiolate)(NC5H4-X) and MeReO(dithiolate)(PAr3) complexes: The equilibrium constants follow the Hammett equation but the rate constants do not
Shan, Xiaopeng,Espenson, James H.
, p. 3612 - 3616 (2003)
Equilibration occurs among the species M...
Efficient Chemoselective Reduction of N-Oxides and Sulfoxides Using a Carbon-Supported Molybdenum-Dioxo Catalyst and Alcohol
Li, Jiaqi,Liu, Shengsi,Lohr, Tracy L.,Marks, Tobin J.
, p. 4139 - 4146 (2019)
The chemoselective reduction of a wide r...
-
Feely,Beavers
, p. 4004,4005 (1959)
-
Mild and efficient method for the synthesis of nitriles
Coskun, Necdet
, p. 1625 - 1630 (2004)
The treatment of aldoximes with a mixtur...
A Versatile VMPO Catalyst Prepared In Situ for Oxidative Ammonolysis of Isomeric Picolines and Xylenes
Dutta, P.,Pathak, D. D.,Senapati, Rabinarayan
, p. 292 - 298 (2020)
Abstract: The V2O5–MoO3–P2O5 (VMPO) cata...
Cyclic Hydroxamic Acid Analogues of Bacterial Siderophores as Iron-Complexing Agents prepared through the Castagnoli–Cushman Reaction of Unprotected Oximes
Bakulina, Olga,Bannykh, Anton,Dar'in, Dmitry,Krasavin, Mikhail
, p. 17667 - 17673 (2017)
The first application of multicomponent ...
Oxidative ammonolysis of 3- and 4-methylpyridines on vanadium oxide catalysts modified with titanium and tin oxides
Mikhailovskaya,Yugay,Chukhno,Sembaev
, p. 191 - 195 (2012)
Catalytic properties of vanadium-titaniu...
Electrochemical Deoxygenation of N-Heteroaromatic N -Oxides
Xu, H.-C.,Xu, P.
, p. 1219 - 1221 (2019)
An electrochemical method for the deoxyg...
Mild Deoxygenation of Sulfoxides over Plasmonic Molybdenum Oxide Hybrid with Dramatic Activity Enhancement under Visible Light
Kuwahara, Yasutaka,Yoshimura, Yukihiro,Haematsu, Kohei,Yamashita, Hiromi
, p. 9203 - 9210 (2018)
Harvesting solar light to boost commerci...
Oxidative ammonolysis of 2,4,6-collidine at vanadium-titanium oxide catalyst
Kagarlitsky,Krichevsky
, p. 315 - 317 (2003)
4-Cyanopyridine was synthesized from the...
A mild and selective deoxygenation of N-oxides with ammonium formate as a catalytic hydrogen transfer agent
Balicki, Roman,Maciejewski, Grzegorz
, p. 1681 - 1683 (2002)
Ammonium formate catalytic transfer hydr...
Photocatalytic deoxygenation of N-O bonds with rhenium complexes: From the reduction of nitrous oxide to pyridineN-oxides
Anthore-Dalion, Lucile,Cantat, Thibault,Kjellberg, Marianne,Nicolas, Emmanuel,Ohleier, Alexia,Thuéry, Pierre
, p. 10266 - 10272 (2021)
The accumulation of nitrogen oxides in t...
-
Afanas'eva et al.
, (1969)
-
-
Prijs et al.
, p. 571,574 (1948)
-
Seeking the Ideal Dehydrating Reagent
Hendrickson, James B.,Hussoin, Md. Sajjat
, p. 4137 - 4139 (1987)
A set of "phosphonium anhydride"reagents...
Oxidative ammonolysis of 3(4)-methyl- and 3,4-dimethylpyridines using vanadium oxide catalysts
Vorobyev,Serebryanskaya
, p. 1987 - 1993 (2012)
Oxidative ammonolysis of 3(4)-methyl- an...
Indium-mediated deoxygenation of amine-N-oxides in aqueous media
Yadav,Subba Reddy,Reddy, M. Muralidhar
, p. 2663 - 2665 (2000)
Several aromatic and aliphatic amine-N-o...
Metal-Free Deoxygenation of Amine N-Oxides: Synthetic and Mechanistic Studies
Lecroq, William,Schleinitz, Jules,Billoue, Mallaury,Perfetto, Anna,Gaumont, Annie-Claude,Lalevée, Jacques,Ciofini, Ilaria,Grimaud, Laurence,Lakhdar, Sami
, p. 1237 - 1242 (2021/06/01)
We report herein an unprecedented combin...
Clean protocol for deoxygenation of epoxides to alkenes: Via catalytic hydrogenation using gold
Fiorio, Jhonatan L.,Rossi, Liane M.
, p. 312 - 318 (2021/01/29)
The epoxidation of olefin as a strategy ...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego