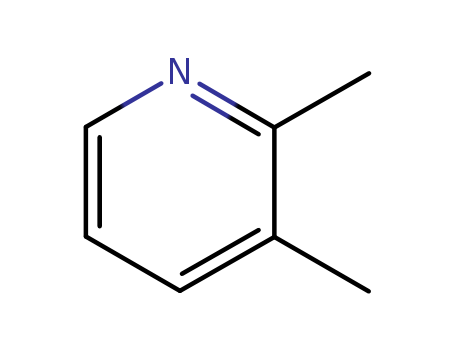
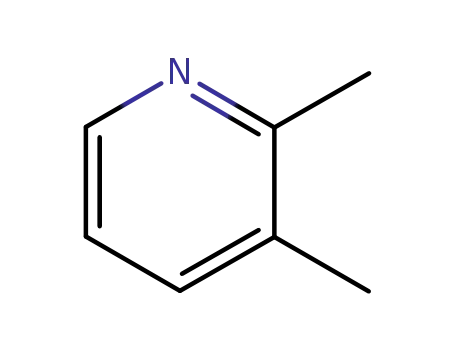
Hot Sale Factory Supply 2,3-Lutidine 583-61-9 with Efficient Delivery
- Molecular Formula:C7H9N
- Molecular Weight:107.155
- Appearance/Colour:clear colorless to slightly yellow liquid
- Vapor Pressure:2.01E-12mmHg at 25°C
- Melting Point:-15 °C(lit.)
- Refractive Index:n20/D 1.508(lit.)
- Boiling Point:160.7 °C at 760 mmHg
- PKA:6.57(at 25℃)
- Flash Point:45.4 °C
- PSA:12.89000
- Density:0.93 g/cm3
- LogP:1.69840
2,3-Lutidine(Cas 583-61-9) Usage
|
Purification Methods
|
Steam distil it from a solution containing about 1.2 equivalents of 20% H2SO4, until ca 10% of the base has been carried over with the non-basic impurities. The acidic solution is then made alkaline, and the base is separated, dried over NaOH or BaO, and fractionally distilled. The distilled lutidine is converted to its urea complex by stirring 100g with 40g of urea in 75mL of H2O, cooling to 5o, filtering at the pump, and washing with 75mL of H2O. The complex, dissolved in 300mL of H2O, is steam distilled until the distillate gives no turbidity with a little solid NaOH. The distillate is then treated with excess solid NaOH, and the upper layer is removed: the aqueous layer is then extracted with diethyl ether. The upper layer and the ether extract are combined, dried (K2CO3), and distilled through a short column. Final purification is by fractional crystallisation using partial freezing. The picrate crystallises from EtOH with m 187-188o. [Kyte et al. J Chem Soc 4454 1960, Beilstein 20 H 243, 20 II 159, 20 III/IV 2765, 20/6 V 15.]
|
InChI:InChI=1/C22H17IN2/c23-21-24-16-17-25(21)22(18-10-4-1-5-11-18,19-12-6-2-7-13-19)20-14-8-3-9-15-20/h1-17H
583-61-9 Relevant articles
Generation and reactions of pyridyllithiums via Br/li exchange reactions using continuous flow microreactor systems
Nagaki, Aiichiro,Yamada, Daisuke,Yamada, Shigeyuki,Doi, Masatomo,Ichinari, Daisuke,Tomida, Yutaka,Takabayashi, Naofumi,Yoshida, Jun-Ichi
, p. 199 - 207 (2013/03/28)
A continuous flow microreactor method fo...
Promiscuous G-protein compositions and their use
-
, (2008/06/13)
Disclosed are compositions and methods f...
Nucleophilic addition to 3-substituted pyridinium salts: Expedient syntheses of (-)-L-733,061 and (-)-CP-99,994
Lemire, Alexandre,Grenon, Michel,Pourashraf, Mehrnaz,Charette, Andre B.
, p. 3517 - 3520 (2007/10/03)
(Chemical Equation Presented) The additi...
Inhibitors of cell proliferation, angiogenesis, fertility, and muscle contraction
-
, (2008/06/13)
The invention concerns inhibitors of cel...
583-61-9 Process route
-
- 50-00-0,30525-89-4,61233-19-0
formaldehyd
-
- 75-07-0,9002-91-9
acetaldehyde
-
- 536-78-7,151103-56-9
3-ethylpyridine
-
- 108-99-6
3-Methylpyridine
Conditions
| Conditions |
Yield |
|
With diammonium phosphate; In ethanol; water; at 234 ℃; for 1h;
|
22%
13%
8%
25% |
-
- 87851-05-6
2-Methyl-3-acetoxymethylpyridin
Conditions
| Conditions |
Yield |
|
With hydrogen; Pd-BaSO4; In ethanol; for 0.5h; Ambient temperature;
|
99.2% |
583-61-9 Upstream products
-
412320-62-8
5,6-dimethyl-1,2,3,4-tetrahydro-pyridine
-
72093-13-1
6-chloro-2,3-dimethylpyridine
-
91324-25-3
1-Methyl-3-acetylpiperidine
-
101252-84-0
4,6-dichloro-2,3-dimethyl-pyridine
583-61-9 Downstream products
-
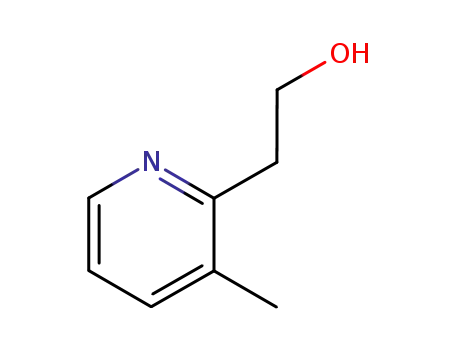
4723-26-6
2-(2-hydroxyethyl)-3-methylpyridine
-
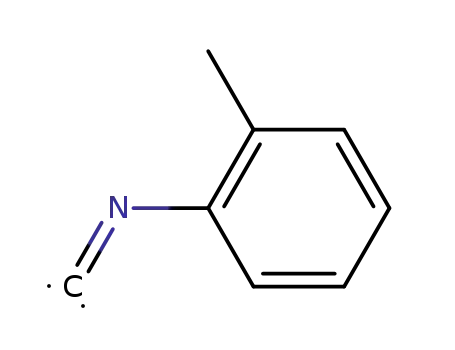
10468-64-1
o-tolyl isocyanide
-
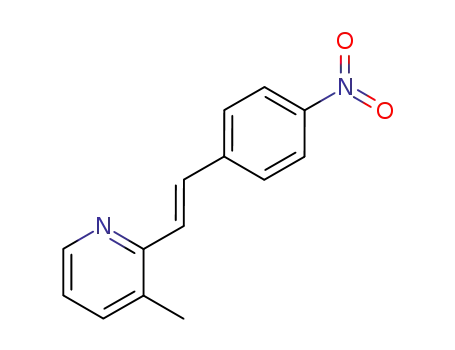
128616-11-5
3-methyl-2-(4-nitro-trans-styryl)-pyridine
-
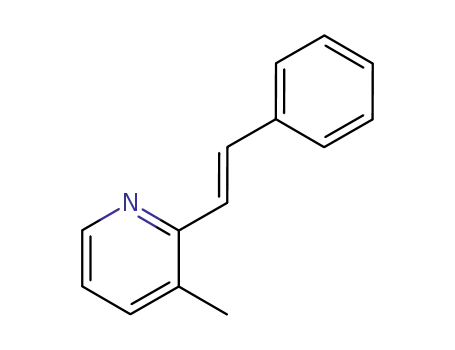
7376-25-2
3-methyl-2-[(E)-2-phenylethenyl]pyridine
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego