|
Preparation
|
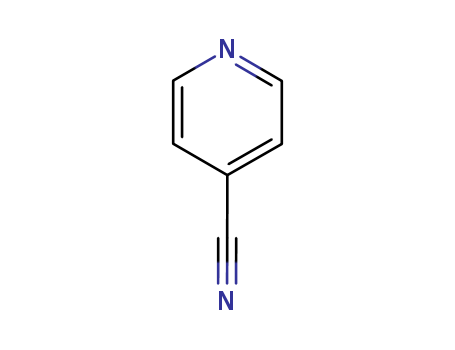
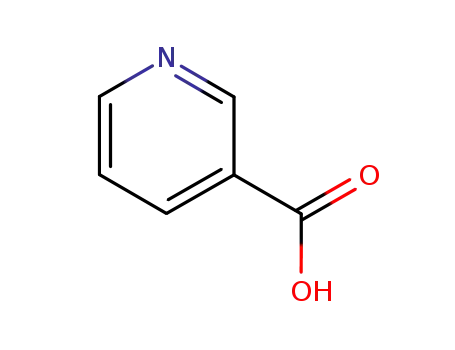
Nicotinic acid exists naturally in grain germs, meats and peanuts. It can also be synthesized artificially through the liquid phase method (potassium permanganate oxidation and nitric acid oxidation) and gas phase method (ozone oxidation, ammonia oxidation and air oxidation). 3-methyl pyridine method In the gas phase ammonia oxidation process, add 3-methyl pyridine, air and ammonia into the fluidized bed reactor and catalyze the reaction at 290~360℃,V2O5 to produce nicotinonitrile; then hydrolyze in sodium hydroxide aqueous solution at 160℃ to produce sodium nicotinate; finally, add hydrochloric acid to acidify, creating nicotinic acid. In the potassium permanganate oxidation method, add potassium permanganate gradually at 80℃ to a mixture of 3-methyl pyridine and water, and then continue to mix for 30min at 85~90℃. Distill to collect and reuse the unreacted 3-methyl pyridine and filter away the produced manganese dioxide. Adjust the PH of the resulting nicotinic acid solution to 3.8~4.0 using hydrochloric acid, cool to 30℃ crystals, and filter to obtain crude nicotinic acid. Dissolve the crude nicotinic acid in hot water, add activated charcoal to eliminate the color, filter, cool, and obtain the crystalline end product. Yield is approximately 86%. 6- hydroxyquinoline method Add sulfuric acid and quinoline into a reaction kettle and mix while maintaining heat at 150~160℃ for 5h. Then with the temperature maintained at 180~220℃, slowly drop in nitric acid and the sulfuric acid mixture over the course of 36~40h. While maintaining the temperature, mix for 2~3h to obtain a nicotinic acid solution and add water to dilute the solution. Use 30%~33% NaOH solution to neutralize the PH to 8~9. Cool and filter away the sodium sulfate and sodium nitrate crystals, add copper sulfate solution to the filtered liquid, and mix and heat to yield copper nicotinate precipitation. Cool, filter and add the copper nicotinate to an adequate amount of water, drop in NaOH solution until PH>9 and the liquid is no longer blue, and filter away the produced cupric oxide. Add a small amount of sodium sulfide solution to remove traces of copper and iron until the solution no longer produces black precipitate, and then filter. Use hydrochloric acid to adjust the PH of the filtered liquid to 3.5~3.9, filter to yield crystals as crude nicotinic acid. Dissolve the crude product in 12 times the amount of distilled water, add activated charcoal to eliminate the color, filter, cool, and obtain the crystalline end product. Yield is 35%~39%. 2-methyl-5-ethyl pyridine method With 2-methyl-5-ethyl pyridine as the raw ingredient, oxidize with nitic acid under high pressure and high temperatures, then decarboxylate to yield nicotinic acid.
|
|
Identifying tests
|
Add 2 portions of 2, 4-Dinitrochlorobenzene to the sample and process into powder. Place 10mg of the powder in a test tube, gently heat until melted, and continue to heat for a couple of seconds. Cool and add 3ml potassium hydroxide ethanol solution (TS-190). The solution should be dark red. Dissolve 50mg of the sample solution in 20ml water, use 0.1mol/L sodium hydroxide to neutralize until a litmus paper reads neutral, and add 3ml copper sulfate solution (TS-78). Blue precipitate should begin forming slowly. Dry the sample for 1h at 105℃ and collect its mineral oil dispersions. The peak wavelength of its infrared absorption spectrum should resemble the standard reference sample formulated using the same method. Prepare an aqueous solution of the sample with a density of 20μg/ml, measure its absorbance at the wavelengths 237nm and 262nm in a 1cm pool, using water as a blank control. A237/A262 should be 0.35~0.39.
|
|
Content analysis
|
Precisely take a sample of 300mg and dissolve in 50ml water. Add a couple drops of phenolphthalein solution (TS-167) and titrate using 0.1mol/L sodium hydroxide. Conduct a control experiment at the same time. Every Ml0.1mol/L sodium hydroxide is equivalent to 12.31mg nicotinic acid (C6H5NO2).
|
|
Toxicity
|
LD50 7.0g/kg (Large mice, oral). GRAS(FDA,§182.5530,2000)。 ADI has no special regulations (EEC, 1990).
|
|
History
|
Huber first synthesized nicotinic acid in 1867. In 1914, Funk isolated nicotinic acid from rice polishings. Goldberger, in 1915, demonstrated that pellagra is a nutritional deficiency. In 1917, Chittenden and Underhill demonstrated that canine blacktongue is similar to pellagra. In 1935, Warburg and Christian showed that niacinamide is essential in hydrogen transport as diphosphopyridine nucleotide (DPN). In the following year, Euler et al. isolated DPN and determined its structure. In 1937, Elvhehjem et al. cured blacktongue by administration of niacinamide derived from liver. In the same year, Fouts et al. cured pellagra with niacinamide. In 1947, Handley and Bond established conversion of tryptophan to niacin by animal tissues.
|
|
Air & Water Reactions
|
Water soluble.
|
|
Reactivity Profile
|
Nicotinic acid is incompatible with strong oxidizers. Nicotinic acid is also incompatible with sodium nitrite.
|
|
Fire Hazard
|
Flash point data for Nicotinic acid are not available; however, Nicotinic acid is probably combustible.
|
|
Biological Activity
|
Nicotinic acid can be converted to nicotinamide in the animal body and, in this form, is found as a component of two oxidation-reduction coenzymes, NAD and NADP.The nicotinamide portion of the coenzyme transfers hydrogens by alternating between an oxidized quaternary nitrogen and a reduced tertiary nitrogen. Enzymes that contain NAD or NADP are usually called dehydrogenases. They participate in many biochemical reactions of lipid, carbohydrate, and protein metabolism. An example of an NAD-requiring system is lactic dehydrogenase which catalyzes the conversion of lactic acid to pyruvic acid.
|
|
Biochem/physiol Actions
|
Nicotinic is an antioxidant and acts as a coenzyme in the form of nicotinamide adenine nucleotides(NAD). It modulates lipid metabolism and may be useful in treating dyslipidemia. Nicotinic acid reduces the low-density lipoprotein (LDL) synthesis and improves high-density lipoprotein (HDL) levels. Deficiency of niacin leads to enhanced lipid peroxidation and is implicated in Crohn′s disease Deficiency also impacts DNA repair and also leads to skin and gastrointestinal disorder pellagra.
|
|
Mechanism of action
|
Nicotinic acid decreases formation and secretion of VLDL by the liver.This action appears secondary to its ability to inhibit fatty acid mobilization from adipose tissue. Circulating free fatty acids provide the main source of fatty acids for hepatic triglyceride synthesis, and lowering triglyceride synthesis lowers VLDL formation and secretion by the liver. Since plasma VLDL is the source of LDL, lowering VLDL can ultimately lower LDL. In addition, nicotinic acid shifts LDL particles to larger (more buoyant) sizes. The larger LDL particles are thought to be less atherogenic. Nicotinic acid can also significantly increase plasma HDL levels; the mechanism is unknown.
|
|
Pharmacokinetics
|
Nicotinic acid is readily absorbed. Peripheral vasodilation is seen within 20 minutes, and peak plasma concentrations occur within 45 minutes. The half-life of the compound is approximately one hour, thus necessitating frequent dosing or an extended-release formulation. Extended release tablets produce peripheral vasodilation within 1 hour, reach peak plasma concentrations within 4 to 5 hours, and have a duration of 8 to 10 hours. Dosing of nicotinic acid should be titrated to minimize adverse effects. An initial dose of 50 to 100 mg t.i.d. often is used with immediate�release tablets. The dose then is gradually increased by 50 to 100 mg every 3 to 14 days, up to a maximum of 6 g/day, as tolerated. Therapeutic monitoring to assess efficacy and prevent toxicity is essential until a stable and effective dose is reached. Similar dosing escalations are available for extended-release products, with doses normally starting at 500 mg once daily at bedtime..
|
|
Side effects
|
Compliance with nicotinic acid therapy can be poor because the drug can produce an intense cutaneous flush. This can be reduced by beginning the drug in stepped doses of 250 mg twice daily and increasing the dose monthly by 500 to 1000 mg per day to a maximum of 3000 mg per day.Taking nicotinic acid on a full stomach (end of meal) and taking aspirin before dosage can reduce the severity of flushing. Time-release forms of nicotinic acid may also decrease cutaneous flushing. Nicotinic acid can cause gastrointestinal (GI) distress,liver dysfunction (especially at high doses), decreased glucose tolerance, hyperglycemia, and hyperuricemia. Thus, it is contraindicated in patients with hepatic dysfunction, peptic ulcer, hyperuricemia, or diabetes mellitus. A paradox associated with nicotinic acid is that it is the most widely available hypolipidemic drug (it is sold over the counter), yet its use requires the closest management by the physician.
|
|
Safety Profile
|
Poison by intraperitoneal route. Moderately toxic by ingestion, intravenous, and subcutaneous routes. Human systemic effects: change in clotting factors, changes in platelet count. Questionable carcinogen with experimental carcinogenic data. When heated to decomposition it emits toxic fumes of NOx.
|
|
Synthesis
|
Nicotinic acid, pyridine-3-carboxylic acid (20.2.9) is synthesized industrially by heating a paraldehyde trimer of acetaldehyde, under pressure with ammonia, which leads to the formation of 2-methyl-5-ethylpyridine, followed by oxidation with nitric acid which gives the desired product.
|
|
Metabolism
|
Nicotinic acid is a B-complex vitamin that is converted to nicotinamide, NAD+ , and NADP+ .The latter two compounds are coenzymes and are required for oxidation/reduction reactions in a variety of biochemical pathways. Additionally, nicotinic acid is metabolized to a number of inactive compounds, including nicotinuric acid and N-methylated derivatives. Normal biochemical regulation and feedback prevent large doses of nicotinic acid from producing excess quantities of NAD+ and NADP+ .Thus, small doses of nicotinic acid, such as those used for dietary supplementation, will be primarily excreted as metabolites, whereas large doses, such as those used for the treatment of hyperlipoproteinemia, will be primarily excreted unchanged by the kidney.
|
|
Purification Methods
|
Crystallise the acid from *benzene, EtOH or H2O. It sublimes without decomposition. [McElvain Org Synth Coll Vol I 385 1941, Beilstein 22 III/IV 439, 22/2 V 57.]
|
|
Applications and Importance
|
Biological, Nutritional, and Food Safety Applications: Nicotinic acid (NA) serves as a basic vitamin B3.
|
|
Antiatherosclerotic Activity
|
Prevention and Treatment of Atherosclerosis: Nicotinic acid (niacin) has been utilized for decades for this purpose.
Antidyslipidemic Effects: Its antiatherogenic activity is believed to result from these effects.
|
|
Dual Potency
|
Vitamin Potency: Effective in milligram doses as a vitamin.
Lipid Drug Potency: Potent in gram doses as a broad-spectrum lipid drug.
|
|
Historical Background
|
Discovery: In the 20th century, Rudolf Altschul discovered the cholesterol-lowering effects of nicotinic acid.
Vitamin Effects: Demonstrated in the early 20th century.
Lipid Effects: Altschul's studies showed lipid-lowering effects, including inhibition of lipid deposits and atheromatosis.
|
|
Mechanism of Action
|
Inhibition of Lipolysis: Nicotinic acid inhibits fat-mobilizing lipolysis in adipose tissue, leading to a decrease in plasma-free fatty acids (FFA).
Coenzyme Precursor: Nicotinic acid and nicotinamide are precursors to the coenzyme nicotinamide adenine dinucleotide (NAD+), crucial in fuel metabolite oxidation.
|
|
Pharmacological Effects
|
Lipid and Lipoprotein Modulation: Pharmacological doses induce significant changes in plasma lipid and lipoprotein levels.
HDL Cholesterol Increase: Nicotinic acid strongly increases plasma high-density lipoprotein (HDL) cholesterol levels.
|
|
Cardiovascular Benefits
|
Atherosclerosis Progression Reduction: Nicotinic acid alone or in combination with LDL cholesterol-lowering drugs can reduce atherosclerosis progression and cardiovascular event risk.
Receptor Discovery: The identification of the G protein鈥揷oupled receptor GPR109A has enhanced our understanding of nicotinic acid's effects.
|
|
Physical properties
|
Nicotinic acid and nicotinamide are colorless crystalline substances. Each is insol uble or only sparingly soluble in organic solvents. Nicotinic acid is slightly soluble in water and ethanol; nicotinamide is very soluble in water and moderately soluble in ethanolNicotinic acid is amphoteric and forms salts with acids as well as bases. Its car boxyl group can form esters and anhydrides and can be reduced. Both nicotinic acid and nicotinamide are very stable in dry form, but in solution nicotinamide is hydro lyzed by acids and bases to yield nicotinic acThe coenzyme forms of niacin are the pyridine nucleotides, NAD(H) and NADP(H). In each of these compounds, the electron-withdrawing effect of the N-1 atom and the amide group of the oxidized pyridine nucleus enables the pyridine C-4 atom to react with many nucleophilic agents (e.g., sulfite, cyanide, and hydride ions). It is the reaction with hydride ions (H?) that is the basis of the enzymatic hydrogen transfer by the pyridine nucleotides; the reaction involves the transfer of two electrons in a single stepSeveral substituted pyridines are antagonists of niacin in biological systems: pyridine-3-sulfonic acid, 3-acetylpyridine, isonicotinic acid hydrazine, 17 and 6-aminonicotinamide
|
|
Application
|
Nicotinic acid is a precursor of the coenzymes NAD and NADP. Widely distributed in nature; appreciable amounts are found in liver , fish, yeast and cereal grains. It is a water-soluble b-complex vitamin that is necessary for the growth and health of tissues. Dietary deficiency is associated with pellagra. It was functions as a nutrient and dietary supplement that prevents pellagra. The term "niacin" has also been applied. The term “niacin” has also been applied to nicotinamide or to other derivatives exhibiting the biological activity of nicotinic acid.
|
|
Definition
|
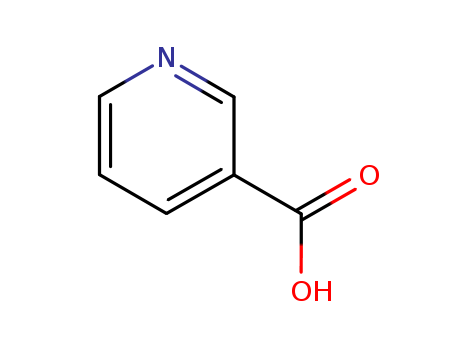
ChEBI: Nicotinic acid is a pyridinemonocarboxylic acid that is pyridine in which the hydrogen at position 3 is replaced by a carboxy group. It has a role as an antidote, an antilipemic drug, a vasodilator agent, a metabolite, an EC 3.5.1.19 (nicotinamidase) inhibitor, an Escherichia coli metabolite, a mouse metabolite, a human urinary metabolite and a plant metabolite. It is a vitamin B3, a pyridinemonocarboxylic acid and a pyridine alkaloid. It is a conjugate acid of a nicotinate.
|
|
Brand name
|
Niacor (Upsher Smith); Niaspan (KOS); Nicolar (Sanofi Aventis); Wampocap (Medpointe).
|
|
General Description
|
Odorless white crystalline powder with a feebly acid taste. pH (saturated aqueous solution) 2.7. pH (1.3% solution) 3-3.5.
|
|
Consumer Uses
|
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment.
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego