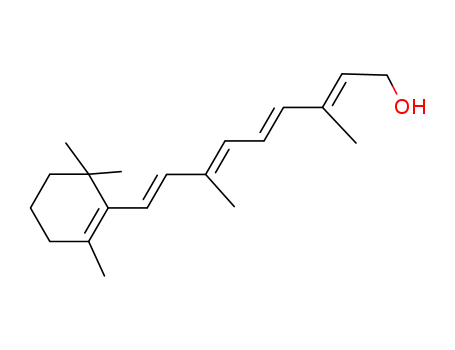|
History
|
The vitamin research is the great achievement in the development of life sciences, while human beings only took half a century to discover and understand vitamins. However, everything is still very difficult for scientists in the early stage of vitamin discovery. From 1913 to 1915, Elmer McCollum and Marguerite Davis indicated that the growth rate was maintained by at least two different kinds of growth factors: one can be separated from eggs or butter, and the other one which multiple neuritis of chicks and pigeons can be extracted by water; thus they were named fat-soluble vitamin A and water-soluble vitamin B.preventedIn 1919, the researchers demonstrated that fat-soluble vitamin A not only sup ported the rate of growth but also prevented eye dryness and night blindness in the process of property study. In 1920, Dr. J.C. Drummond named this active lipid as vitamin A. It exists in cod liver oil and prevents the occurrence of eye dryness and night blindness.
|
|
Indications
|
Vitamin A, or retinol, is essential for the proper maintenance of the functional and structural integrity of epithelial cells, and it plays a major role in epithelial differentiation. Bone development and growth in children have also been linked to adequate vitamin A intake. Vitamin A, when reduced to the aldehyde 11-cis-retinal, combines with opsin to produce the visual pigment rhodopsin. This pigment is present in the rods of the retina and is partly responsible for the process of dark adaptation.
|
|
Manufacturing Process
|
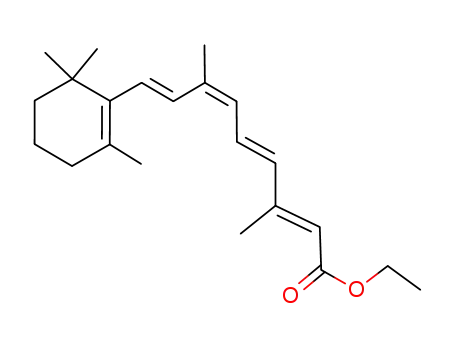
Manufacturing process for Vitamin A includes these steps as follows: Step A: Synthesis of Preparation of ethyl ether of ethynyl-β-ionol;Step B: Coupling Reaction; Step C:Semi-Hydrogenation of Coupling Product;Step D:Hydrolysis of Semi-Hydrogenated Coupling Product. Separation of Vitamin A from the product obtained was achieved by acetylating the total reaction product using pyridine-acetic anhydride at room temperature and chromatographing on alumina neutralized with acetic acid. A fairly clean separation was achieved. The Vitamin A acetate fraction was sufficiently pure to become crystallized from pentane at -15°C when seeded with a pure Vitamin A acetate crystal. When the Vitamin A acetate was converted to the alcohol form of Vitamin A, the final product showed the characteristic infrared and ultraviolet absorption curves for Vitamin A. Similar results were obtained using as co-solvents (with the liquid ammonia) ethylene diamine and ether; pentane; tetrahydrofuran; diethylamine and hexamethylphosphoramide.
|
|
World Health Organization (WHO)
|
Vitamin A, a fat-soluble vitamin, is used in the treatment and prevention of vitamin A deficiency resulting from inadequate dietary intake. It has been demonstrated to be teratogenic at high doses (more than 25,000 IU per day). Daily dosages of less than 10000 IU seem to be free of this risk. Retinol (vitamin A) is listed in the WHO Model List of Essential Drugs.
|
|
Synthesis Reference(s)
|
Tetrahedron, 51, p. 2435, 1995 DOI: 10.1016/0040-4020(94)01108-C
|
|
Biochem/physiol Actions
|
Retinol and its derivatives exhibit anti-aging properties. Retinol is used for treating wrinkles and signs of aging. However, due to its photo instability and skin irritation potency, it is hardly used in cosmetic formulations. Retinol is also used as a therapeutic for dermatoses. Its deficiency leads to xerosis and follicular hyperkeratosis.
|
|
Pharmacology
|
Intake of vitamin A precursors, such as carotenoids, retinyl esters, retinol, and reti nal, can maintain the epithelial cell differentiation, normal proliferation, and visual function. All of these substances can be metabolized into retinol, retinal, and reti noic acid. But unlike retinol and retinal, retinoic acid cannot be reduced to retinol and retinal. Intake of retinoic acid can only maintain the systemic function of vita min A.Visual and vitamin A. 11-cis-retinal plays an important role as a photographic group of retinal cones and visual pigments in rod cells. 11-cis-retinal would be transformed into all-trans-retinal form under the light induction. The dissociation of all-trans retinal and opsin was coupled with the nerve stimulation of the brain’s visual center. By a series of biochemical processes, nerve impulses format in the rod cells at the end of synapse, and then the optic nerve conducts the nerve impulses along. The visual process is a component renewable cycle, and all-trans-retinal can be enzymatically modified to 11-cis form in dark conditions.The systemic effects of vitamin A. Vitamin A not only significantly affects visual function but also has a greater physiological impact than visual function. Vitamin A deficiency destroys the visual cycle, leads to dark adaptation damage (night blind ness or nyctalopia), and destroys systemic function which is necessary to maintain life (e.g., corneal injury, infection, and hypoplasia). Vitamin A deficiency can lead to animal death.Vitamin A functions in reproduction and embryonic development. Vitamin A plays an important role in the reproductive process of sperm production and ovula tion, but its biochemical basis is unclear. Vitamin A plays a key role in the develop ment of embryos and organism and maintenance of tissue function. The main organs affected by vitamin A deficiency are the heart, eye tissue, circulatory system, geni tourinary system, and respiratory system. Vitamin A is necessary for embryonic development.Vitamin A functions on immune function. The lymphoid organs, cell distribu tion, histology, lymphocytes, and other characteristics will change when the ani mals lack vitamin A. Vitamin A deficiency can lead to immune function decrease, induce inflammation, and exacerbate inflammatory symptomsVitamin A functions in dermatology. Vitamin A plays an important role in main taining healthy skin. Vitamin A deficiency disrupts human keratin cell terminal dif ferentiation and makes the skin rough, dry, scaly, and clogged It is reported that vitamin A can degrade malignant melanoma and T-cell lymphoma epidermal transfer, reduce the oil secretion of the common acne and the number of bacteria in the epidermis and capillaries, and inhibit immune response of monocytes and neutrophils.Vitamin A plays an important role as an important function material in the body system, such as hematopoietic function, bone development, tumor prevention, and so on. Therefore, supplement of vitamin A is necessary for health requirements
|
|
Side effects
|
Acute hypervitaminosis A results in drowsiness, headache, vomiting, papilledema, and a bulging fontanel in infants. The symptoms of chronic toxicity include scaly skin, hair loss, brittle nails, and hepatosplenomegaly. Anorexia, irritability, and swelling of the bones have been seen in children. Retardation of growth also may occur. Liver toxicity has been associated with excessive vitamin A intake. Vitamin A is teratogenic in large amounts, and supplements should not be given during a normal pregnancy. The IOM has reported the UL of vitamin A to be 3,000 μg/day.
|
|
Safety Profile
|
Moderately toxic by ingestion. Human teratogenic effects by ingestion: developmental abnormalities of the craniofacial area and urogenital system. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
|
|
Purification Methods
|
Purify retinol by chromatography on columns of water-deactivated alumina and elute with 3-5% acetone in hexane. Separate the isomers by TLC plates on silica gel G, developed with pet ether (low boiling)/methyl heptanone (11:2). Store it in the dark, under N2, at 0o, or in Et2O, Me2CO or EtOAc. [See Gunghaly et al. Arch Biochem Biophys 38 75 1952, Beilstein 6 IV 4133.]
|
|
Toxicity evaluation
|
The exact mechanism leading to toxicity is not known. Both acute and chronic toxicity may occur. Acute and Short-Term Toxicity (or Exposure) Human Acute toxicity is uncommon in adults. However, vitamin A ingestions of greater than 1 million IU in adults and greater than 300 000 IU in children have resulted in the development of increased intracranial pressure (symptoms described include headache, dizziness, vomiting, visual changes, and bulging fontanel in infants). Acute ingestions of greater than 12 000 IU per kilogram are also considered toxic. Chronic Toxicity (or Exposure) Human Toxicity is more frequently seen with chronic ingestion of high doses of 30 000–50 000 IU per day. Vitamin A toxicity in children develops following chronic ingestion of 410 times the recommended daily allowance for weeks to months. Malnutrition and individual tolerance may also be factors in predisposition to toxicity. Signs and symptoms of toxicity include vomiting, anorexia, agitation, fatigue, double vision, headache, bone pain, alopecia, skin lesions, increased intracranial pressure, and papilledema. Hepatic toxicity typically requires months or years of daily high doses of vitamin A. There are no known cases of vitamin A toxicity associated with beta-carotene ingestion.
|
|
Physical properties
|
Vitamin A1 (VA1), Molecular formula, C20H30O; MW, 286.45; CAS, 68-26-8. Melting point: 62–64 °C. Boiling point: 137–138 °CVA2, Molecular formula, C20H28O; MW, 284.44; Melting point: 17–19 °C.
|
|
Definition
|
ChEBI: A retinol in which all four exocyclic double bonds have E- (trans-) geometry.
|
|
Brand name
|
Avibon.
|
|
General Description
|
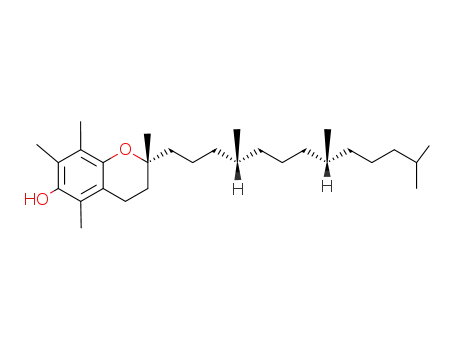
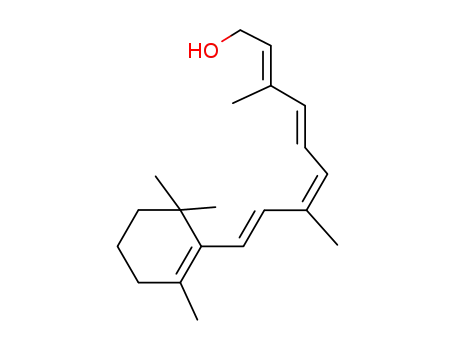
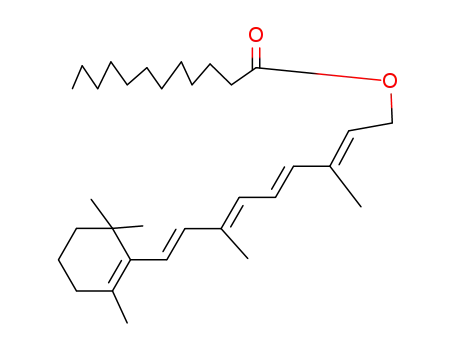
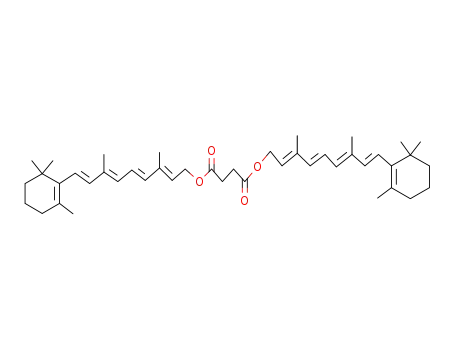
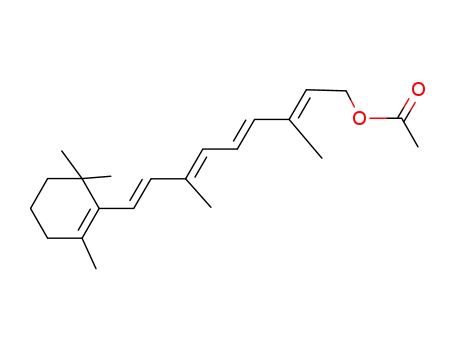
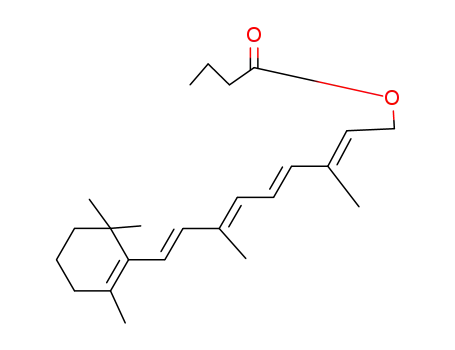
Retinal, retinol and retinoic acid are the aldehyde, alcohol and acid forms of vitamin A. The retinoids exist as many geometric isomers due to the unsaturated bonds in the aliphatic chain. Retinol is biologically active in a wide range of processes.
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego

![O-(4-oxo-4-{[2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2H-chromen-6-yl]oxy}butanoyl)retinol](/upload/2024/6/22e32217-6cd3-4fb5-bbbf-8b42e216e16e.png)