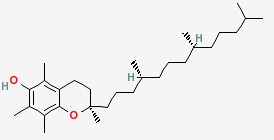
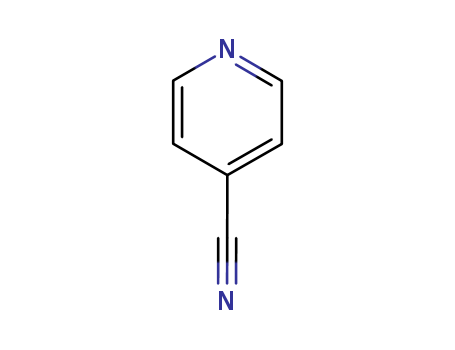
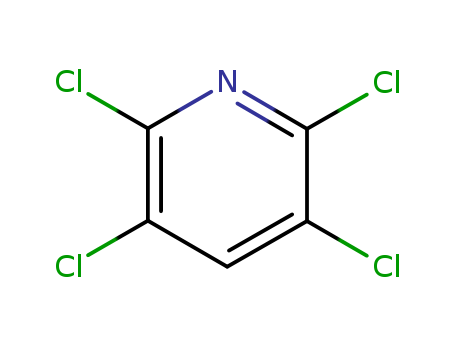
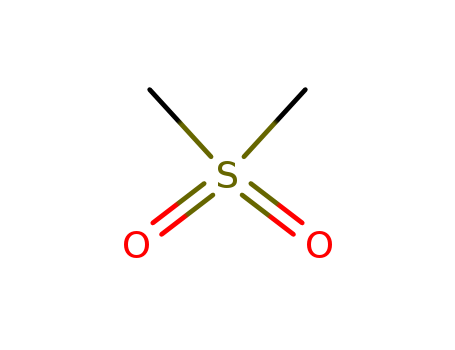
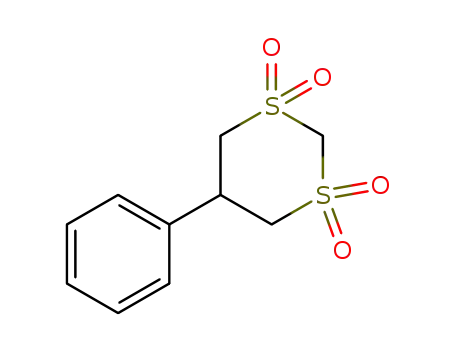
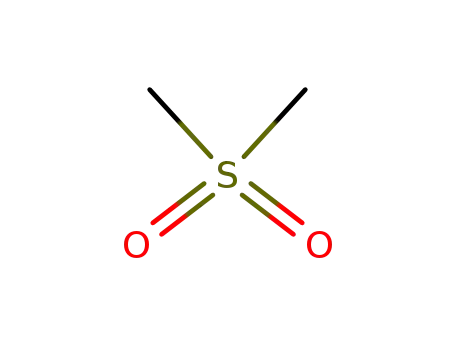
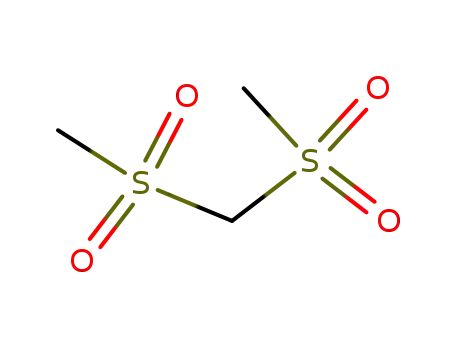
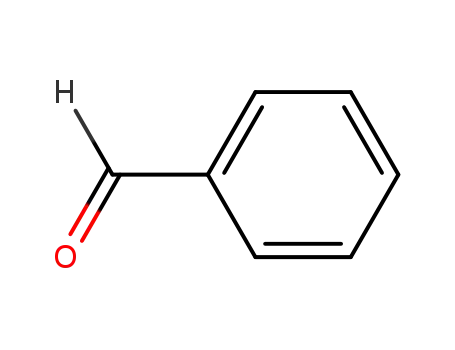
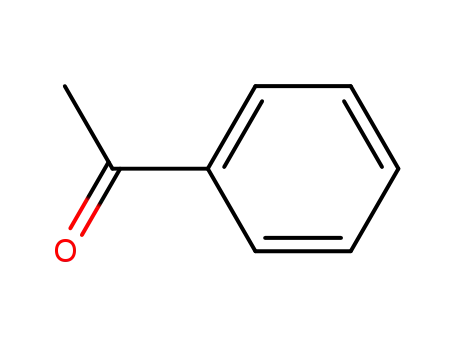
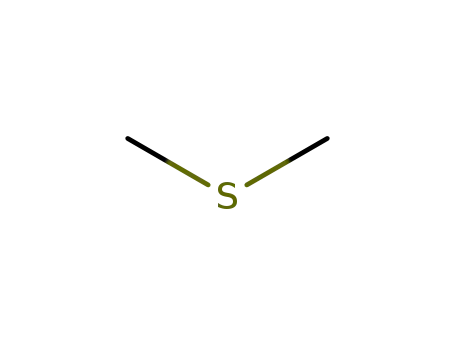
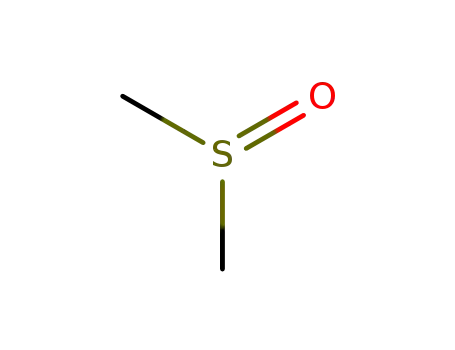
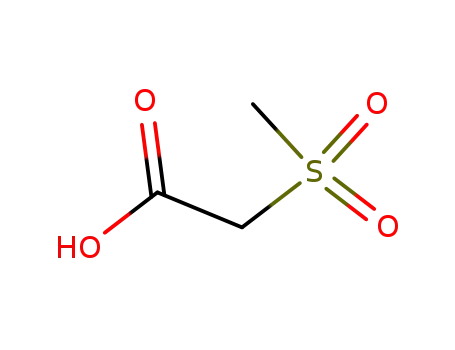
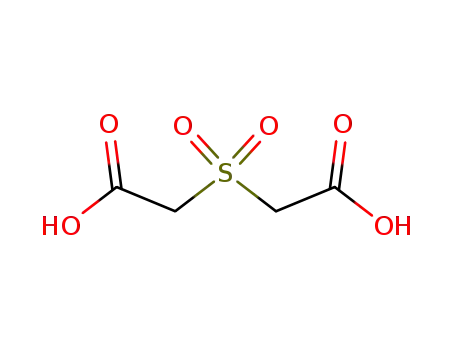
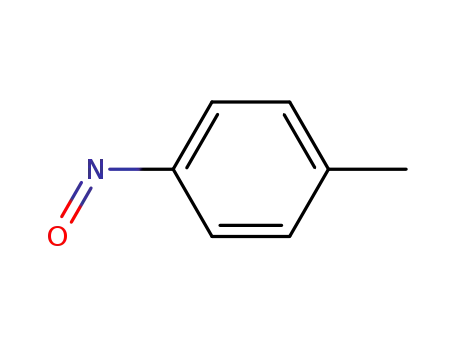
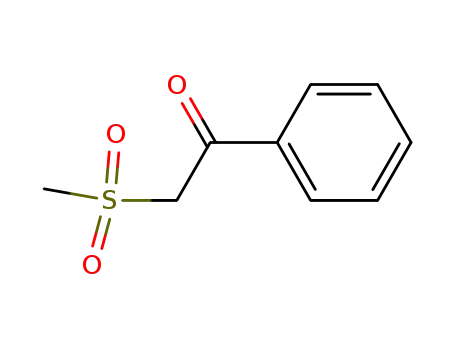
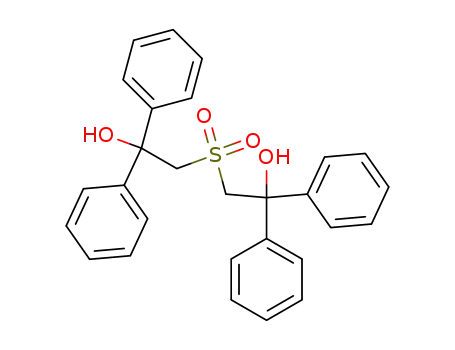
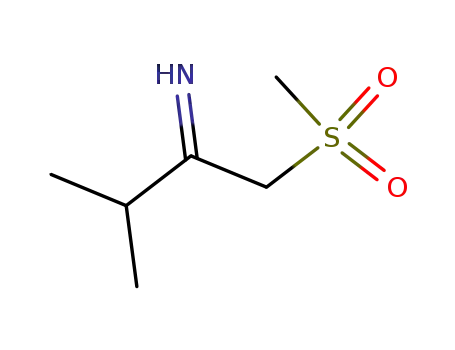
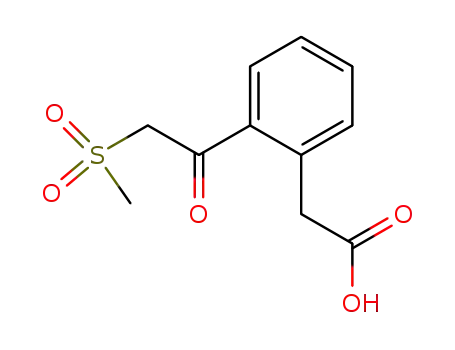
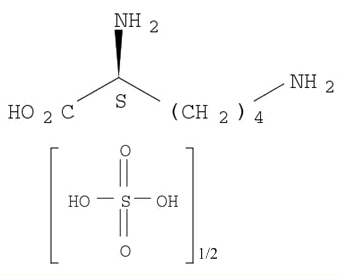
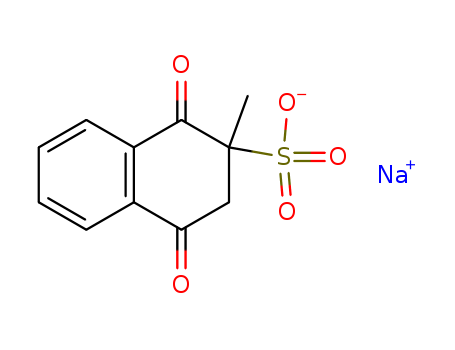
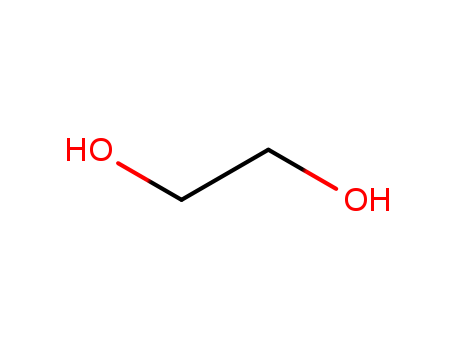
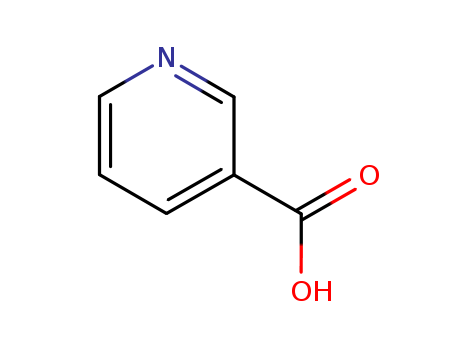
| Conditions |
Yield |
|
With C30H24N2O7W; dihydrogen peroxide; In dichloromethane; water; for 1h; Reflux;
|
6%
94% |
|
With dihydrogen peroxide; In ethanol; hexane; water; at 60 ℃; for 0.0833333h;
|
92%
6% |
|
With dihydrogen peroxide; In water; at 30 - 35 ℃; for 24h; Sealed tube; Green chemistry;
|
68%
62% |
|
With 3-hydroperoxy-3-methyl-1-phenylindolin-2-one; In dichloromethane; for 2h; Heating;
|
24%
49% |
|
With 1,1,4,4-Tetramethyl-2,3-dioxa-1,4-disilacyclohexane; In Petroleum ether; at 50 ℃; for 12h;
|
19%
15% |
|
With tert.-butylhydroperoxide; aluminum tri-tert-butoxide; In benzene;
|
17% |
|
With oxaziridinium salt 1; In [D3]acetonitrile; at 20 ℃;
|
3 % Spectr.
94 % Spectr. |
|
With 3,3-bistrifluoromethyl-2-trifluoromethyl-oxaziridine; In chloroform-d1; trichlorofluoromethane; at -30 ℃; for 0.333333h;
|
0.4 % Spectr.
75 % Spectr. |
|
With bis-[(trifluoroacetoxy)iodo]benzene; In chloroform; at 20 ℃; for 12h;
|
15 % Spectr.
45 % Spectr. |
|
With dihydrogen peroxide; methyltrioxorhenium(VII); In chloroform-d1; at 20 ℃; for 24h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With ozone; In solid matrix; at -263.2 ℃; Mechanism; Irradiation; also methanethiol;
|
|
|
With dihydrogen peroxide; methyltrioxorhenium(VII); In chloroform-d1; at 20 ℃; for 24h; Product distribution; reactions of derivatives under var. conditions;
|
|
|
With dihydrogen peroxide; In water; at 19.9 ℃; Product distribution; Mechanism; Kinetics; other reagents, pH dependence;
|
|
|
With oxaziridinium salt 1; In [D3]acetonitrile; at 20 ℃; for 1.5h;
|
10 % Spectr.
90 % Spectr. |
|
With dihydrogen peroxide; Further Variations:; Temperatures; molar ratio; Product distribution;
|
|
|
With cis-VI(6,6'-Cl2bpy)2O2>; In acetonitrile; at 20 ℃; for 0.5h;
|
68 % Chromat.
9 % Chromat. |
|
With dihydrogen peroxide; at 5 ℃; for 0.166667h; Further Variations:; Temperatures; Product distribution;
|
|
|
With nitric acid; In acetonitrile; at 20 ℃; for 15h; chemoselective reaction;
|
|
|
With dihydrogen peroxide; In methanol; at 20 ℃; for 0.25h; chemoselective reaction;
|
|
|
With dihydrogen peroxide; In acetonitrile; at 20 ℃; for 0.5h;
|
|
|
With C38H26N6NiS2(2+)*2NO3(1-); dihydrogen peroxide; In water; acetonitrile; at 20 ℃;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In chloroform-d1;
|
|
|
With (η5-C5H5)Mo(CO)3C2Ph; dihydrogen peroxide; In acetonitrile; at 20 ℃; for 2h; Inert atmosphere; Schlenk technique;
|
|
|
With dihydrogen peroxide; In acetonitrile; at 20 ℃; for 1h; Catalytic behavior;
|
|
|
With ozone; In acetone; at 60 ℃; for 2h; under 3750.38 Torr; Solvent; Pressure; Temperature; Reagent/catalyst;
|
|
|
With bismuth oxybromide; oxygen; In acetonitrile; for 12h; under 750.075 Torr; Irradiation;
|
|
|
With dihydrogen peroxide; In methanol; at 30 ℃; for 600h; under 1125.11 Torr; Reagent/catalyst; Solvent; Temperature; Molecular sieve;
|
|
|
With dihydrogen peroxide; [Ni0.5(1-vinylimidazole)2]3[Ni0.5(1-vinylimidazole)H2O][V4O12]*H2O; In ethanol; water; at 45 ℃; for 4h;
|
|
|
With water; dihydrogen peroxide; C16H17MoN3O6; In ethanol;
|
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego