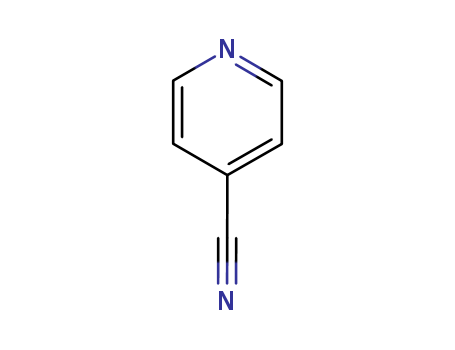
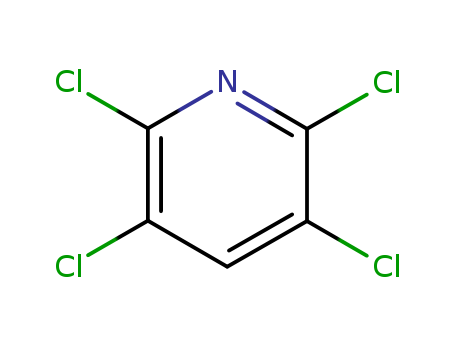
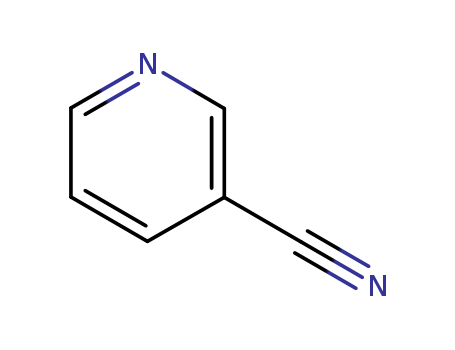
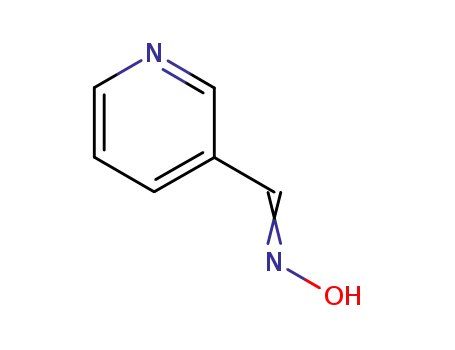
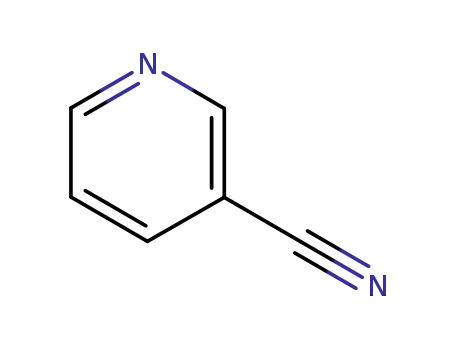
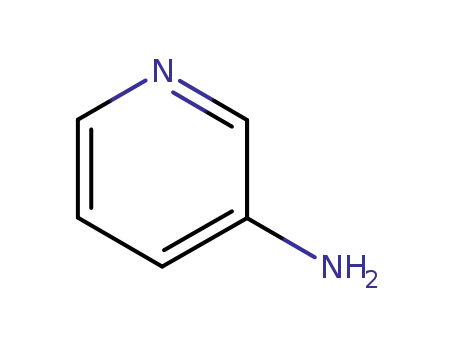
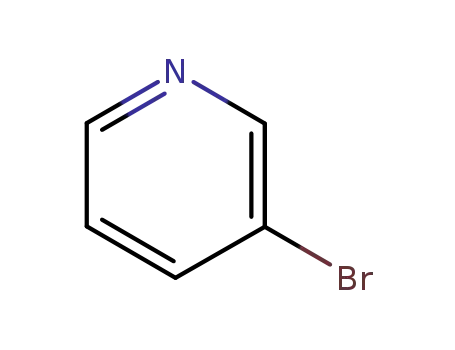
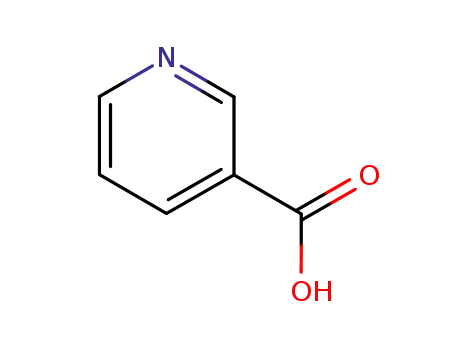
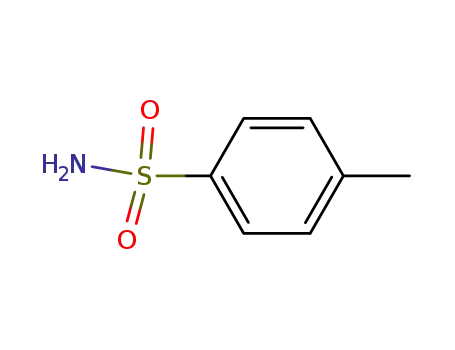
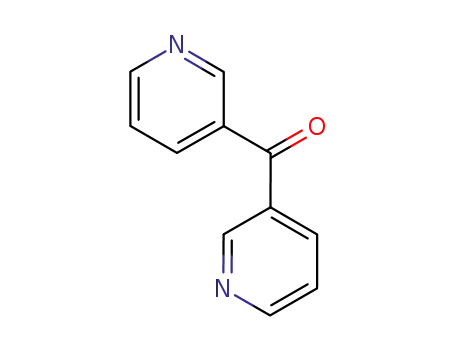
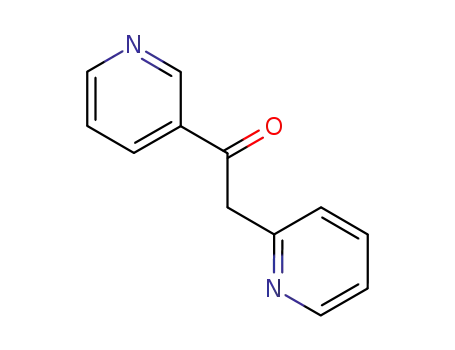
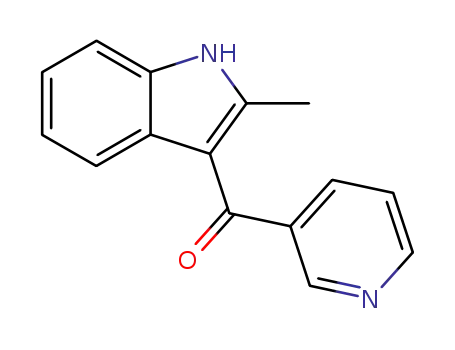
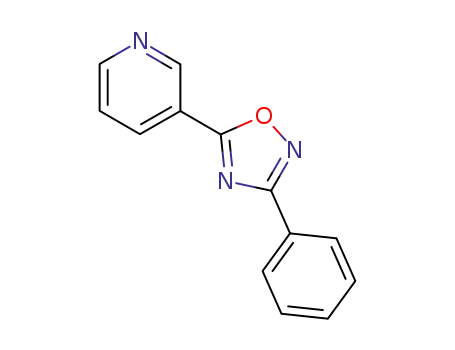
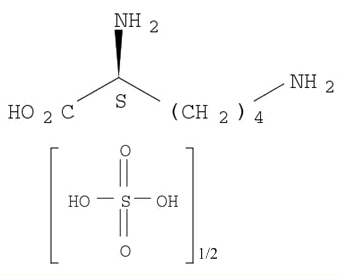
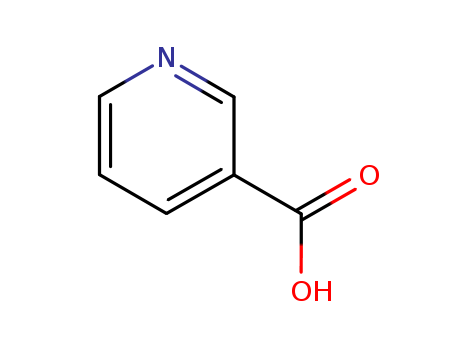
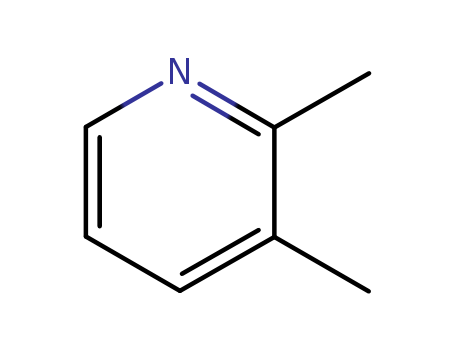
Metalloporphyrin catalyzed oxidation of N-hydroxyguanidines: A biomimetic model for the H2O2-dependent activity of nitric oxide synthase
Keseru, Gyoergy M.,Balogh, Gyoergy T.,Karancsi, Tamas
, p. 1775 - 1777 (2000)
A chemical model for the H2O2 promoted o...
Highly efficient synthesis of primary amides: Via aldoximes rearrangement in water under air atmosphere catalyzed by an ionic ruthenium pincer complex
Yang, Fa-Liu,Zhu, Xinju,Rao, Dun-Kang,Cao, Xiao-Niu,Li, Ke,Xu, Yan,Hao, Xin-Qi,Song, Mao-Ping
, p. 37093 - 37098 (2016)
The transformation of aldoximes to prima...
Rhenium(VII) oxo complexes as extremely active catalysts in the dehydration of primary amides and aldoximes to nitriles
Ishihara, Kazuaki,Furuya, Yoshiro,Yamamoto, Hisashi
, p. 2983 - 2986 (2002)
An economical and environmentally benign...
An indium mediated efficient chemoselective deoxygenation of N-oxides and nitrones
Ilias, Md,Barman, Dhiren C,Prajapati, Dipak,Sandhu, Jagir S
, p. 1877 - 1879 (2002)
A simple and inexpensive procedure for t...
-
Aumann,Deady
, p. 32 (1973)
-
Catalytic conversion of nicotine into nicotinonitrile - A pharmaceutical intermediate product
Kagarlitskii,Iskakova,Turmukhambetov
, p. 26 - 27 (2002)
-
1-Cyanoimidazole as a mild and efficient electrophilic cyanating agent
Wu, Yong-Qian,Limburg, David C.,Wilkinson, Douglas E.,Hamilton, Gregory S.
, p. 795 - 797 (2000)
formula presented A mild and high-yieldi...
Ternary VZrALON oxynitrides - Efficient catalysts for the ammoxidation of 3-picoline
Janke, Christiane,Radnik, J?rg,Bentrup, Ursula,Martin, Andreas,Brückner, Angelika
, p. 2687 - 2695 (2014)
Starting from previous binary VZrON (VAl...
Dispersion and 3-Picoline Ammonoxidation Investigation of V2O5/α-Al2O3 Catalysts
Reddy, Benjaram Narasimha,Reddy, Benjaram Mahipai,Subrahmanyam, Machiraju
, p. 1649 - 1655 (1991)
The effect of changing the precursor on ...
Statistical experimental design-driven discovery of room-temperature conditions for palladium-catalyzed cyanation of aryl bromides
Stazi, Federica,Palmisano, Giovanni,Turconi, Marco,Santagostino, Marco
, p. 1815 - 1818 (2005)
A combination of Pd2(dba)3·CHCl3 (0.5 mo...
-
Woodward et al.
, p. 540,542 (1944)
-
Perrhenic acid-catalyzed dehydration from primary amides, aldoximes, N-monoacylureas, and α-substituted ketoximes to nitrile compounds
Furuya, Yoshiro,Ishihara, Kazuaki,Yamamoto, Hisashi
, p. 400 - 406 (2007)
The dehydration reaction of primary amid...
Iodine/aqueous NH4OAc: An improved reaction system for direct oxidative conversion of aldehydes and alcohols into nitriles
Ren, Yi-Ming,Zhu, Yi-Zhong,Cai, Chun
, p. 18 - 19 (2008)
A convenient method for direct oxidative...
-
Gagnon et al.
, p. 1662,1666 (1956)
-
A Selective and Mild Oxidation of Primary Amines to Nitriles with Trichloroisocyanuric Acid
Chen, Fen-Er,Kuang, Yun-Yan,Dai, Hui-Fang,Lu, Liang,Huo, Ming
, p. 2629 - 2631 (2003)
An efficient and highly selective method...
Exploration of relative chemoselectivity in the hydrodechlorination of 2-chloropyridines
Kinarivala, Nihar,Trippier, Paul C.
, p. 5386 - 5389 (2014)
The chemoselectivity of hydrodechlorinat...
Hydrothermal synthesis of microporous W-V-O as an efficient catalyst for ammoxidation of 3-picoline
Goto, Yoshinori,Shimizu, Ken-Ichi,Murayama, Toru,Ueda, Wataru
, p. 118 - 122 (2016)
W-V complex metal oxide (W-V-O) was prep...
bis(trichloromethyl)carbonate, an efficient activator for the one-pot conversion of aldehydes into nitriles under mild conditions
Bose, D. Subhas,Goud, P. Ravinder
, p. 3621 - 3624 (2002)
A single step conversion of aldehydes in...
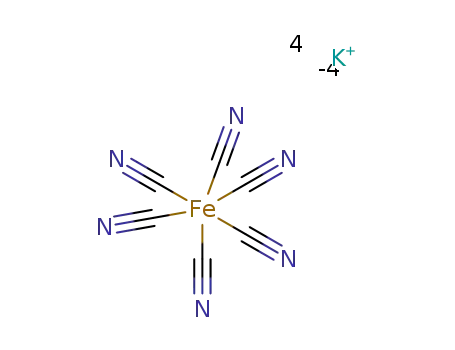
Potassium hexacyanoferrate(II) - A new cyanating agent for the palladium-catalyzed cyanation of aryl halides
Schareina, Thomas,Zapf, Alexander,Beller, Matthias
, p. 1388 - 1389 (2004)
A new advantageous cyanating agent, pota...
Characterization and reactivity of molybdenum oxide catalysts supported on niobia
Chary, Komandur V.R.,Bhaskar, Thallada,Kishan, Gurram,Reddy, Kondakindi Rajender
, p. 4392 - 4399 (2001)
A series of MoO3/Nb2O5 catalysts with Mo...
A simple method for the synthesis of nitriles from primary amides under neutral conditions
Bose, D. Subhas,Sunder, K. Sugnana
, p. 4235 - 4239 (1999)
1-(3-Dimethylaminopropyl)-3-ethylcarbodi...
A Novel Single Step Synthesis of Nicotinamide by β-Picoline Ammoxidation over MoO3 and CuO Oxide Catalysts
Reddy, B. Mahipal,Subrahmanyam, M.
, p. 940 - 941 (1988)
Ammonia up-take has been correlated dire...
TrocCl mediated efficient synthesis of nitriles from primary amides
Bose, D. Subhas,Kumar, K. Kiran
, p. 3047 - 3052 (2000)
In order to establish a rapid conversion...
Decarbonylative Synthesis of Aryl Nitriles from Aromatic Esters and Organocyanides by a Nickel Catalyst
Iizumi, Keiichiro,Kurosawa, Miki B.,Isshiki, Ryota,Muto, Kei,Yamaguchi, Junichiro
, p. 1555 - 1559 (2021)
A decarbonylative cyanation of aromatic ...
A convenient procedure for the palladium-catalyzed cyanation of aryl halides
Sundermeier, Mark,Zapf, Alexander,Beller, Matthias
, p. 1661 - 1664 (2003)
A useful source of cyanide for the palla...
Chemistry of α-Amino Nitriles. Exploratory Experiments on Thermal Reactions of α-Amino Nitriles
Xiang, Yi-Bin,Drenkard, Susanne,Baumann, Karl,Hickey, Deirdre,Eschenmoser, Albert
, p. 2209 - 2250 (1994)
The paper extends a previously published...
Aryl chlorothionoformate: A new versatile reagent for the preparation of nitriles and isonitriles under mild conditions
Subhas Bose,Ravinder Goud
, p. 747 - 748 (1999)
Aryl chlorothionoformate is a very usefu...
-
Trubnikov et al.
, (1969)
-
Transformation of alkaloid anabasin into nicotinonitrile
Iskakova,Kagarlitskii
, p. 656 - 658 (1999)
The possibility of preparing nicotinonit...
-
Sovorov et al.
, (1970)
-
Sulfate additives generate robust and highly active palladium catalysts for the cyanation of aryl chlorides
Shevlin, Michael
, p. 4833 - 4836 (2010)
The use of sulfate additives such as H2S...
Heterocycles from amino acids. A novel synthetic approach for imidazo[1,5-a]pyridines and imidazo[1,5-a]quinolines
Kolar,Petric,Tisler
, p. 1715 - 1720 (1991)
-
Triphenylphosphine-iodine: An efficient reagent system for the synthesis of nitriles from aldoximes
Venkat Narsaiah,Sreenu,Nagaiah
, p. 137 - 140 (2006)
A wide range of aldoximes were smoothly ...
A versatile protocol for copper-catalyzed cyanation of aryl and heteroaryl bromides with acetone cyanohydrin
Schareina, Thomas,Zapf, Alexander,Cotte, Alain,Gotta, Matthias,Beller, Matthias
, p. 777 - 780 (2011)
A novel copper-catalyzed cyanation of ar...
Development of Pd/C-catalyzed cyanation of Aryl halides
Yu, Hannah,Richey, Rachel N.,Miller, William D.,Xu, Jiansheng,May, Scott A.
, p. 665 - 668 (2011)
A practical method for palladium-catalyz...
Ammoxidation of 3-Picoline to Nicotinonitrile over Highly Dispersed V2O5/ZrO2 Catalysts
Chary,Kishan,Narayana,Bhaskar
, p. 314 - 315 (1998)
Vapour phase ammoxidation of 3-picoline ...
Rapid cyanation of aryl iodides in water using microwave promotion
Arvela, Riina K.,Leadbeater, Nicholas E.,Torenius, Hanna M.,Tye, Heather
, p. 1119 - 1121 (2003)
We show that using water in conjunction ...
Synthesis, characterization and catalytic performance of titania supported VPO catalysts for the ammoxidation of 3-picoline
Kalevaru,Madaan,Martin
, p. 52 - 62 (2011)
Series of vanadium phosphorus oxide (VPO...
New method of obtaining nicotinic acid from 2-methyl-5-ethylpyridine
Suvorov,Yakovlev,Kagarlitskii,Guseinov,Kutzhanov,Kan,Lebedeva
, p. 495 - 497 (1977)
-
FTIR study of β-picoline and pyridine-3-carbaldehyde transformation on V-Ti-O catalysts. the effect of sulfate content on β-picoline oxidation into nicotinic acid
Chesalov, Yuriy A.,Andrushkevich, Tamara V.,Sobolev, Vladimir I.,Chernobay, Galina B.
, p. 118 - 130 (2013)
Surface complexes of β-picoline, 3-pyrid...
Studies on the ammoxidation of N heterocyclic compounds. I. Vapor phase ammoxidation of 3 picoline (Japanese)
Okada,Morita,Miwa
, p. 1443 - 1450 (1972)
-
A practical synthesis of highly functionalized aryl nitriles through cyanation of aryl bromides employing heterogeneous Pd/C: In quest of an industrially viable process
Hatsuda, Masanori,Seki, Masahiko
, p. 9908 - 9917 (2005)
Preparation of aryl nitrile 2a through c...
A SIMPLE REDUCTION OF AROMATIC HETEROCYCLIC N-OXIDES WITH HEXAMETHYLDISILANE: REACTIONS WITH HEXAMETHYLDISILANE AND FLUORIDE ION I
Vorbrueggen, Helmut,Krolikiewicz, Konrad
, p. 5337 - 5338 (1983)
Aromatic heterocyclic N-Oxides are readi...
A practical synthesis of highly functionalized aryl nitriles through cyanation of aryl bromides employing heterogeneous Pd/C
Hatsuda, Masanori,Seki, Masahiko
, p. 1849 - 1853 (2005)
An industrially viable cyanation of aryl...
Preparation of nitriles from primary amides under Swern oxidation conditions
Nakajima, Noriyuki,Ubukata, Makoto
, p. 2099 - 2102 (1997)
In order to establish a mild conversion ...
Effect of reaction medium on the composition of vanadium-titanium catalyst in oxidative ammonolysis of alkylpyridines
Saurambaeva,Sembaev
, p. 1104 - 1107 (2002)
The influence exerted by the concentrati...
The Semmler-Wolff Aromatization and Schmidt Reaction Applied to Some Pyridopyrazolobenzotriazines
Kocevar, Marijan,Stanovnik, Branko,Tisler, Miha
, p. 597 - 604 (1988)
Pyridopyrazolobenzotriazin-4(1H)ones wer...
Ammoxidation of 3-picoline to nicotinonitrile using silica-supported VCrO catalysts
Jiang, Feng,Wei, Ruiping,Gao, Lijing,Xiao, Guomin,Niu, Lei
, p. 1353 - 1361 (2013)
A series of vanadium-chromium oxide (VCr...
A facile microwave-assisted palladium-catalyzed cyanation of aryl chlorides
Chobanian, Harry R.,Fors, Brett P.,Lin, Linus S.
, p. 3303 - 3305 (2006)
We report an efficient method for the pr...
Efficient palladium-catalyzed cyanation of aryl/heteroaryl bromides with K4[Fe(CN)6] in t-BuOH-H2O using tris(2-morpholinophenyl)phosphine as a ligand
Zou, Tao,Feng, Xiujuan,Liu, Hesong,Yu, Xiaoqiang,Yamamoto, Yoshinori,Bao, Ming
, p. 20379 - 20384 (2013)
A practical method for the synthesis of ...
Palladium-catalyzed synthesis of nitriles from N-phthaloyl hydrazones
Ano, Yusuke,Chatani, Naoto,Higashino, Masaya,Yamada, Yuki
supporting information, p. 3799 - 3802 (2022/04/07)
The Pd-catalyzed transformation of N-pht...
Bis-morpholinophosphorylchloride, a novel reagent for the conversion of primary amides into nitriles
Rao, P. Purnachandra,Nowshuddin, Shaik,Jha, Anjali,Rao, B. Leela Maheswara,Divi, Murali K.,Rao
supporting information, (2021/01/21)
Bis-morpholinophosphorylchloride (Bmpc),...
Method for dehydrating primary amide into nitriles under catalysis of cobalt
-
Paragraph 0126-0128, (2021/06/21)
The invention provides a method for dehy...
METHOD FOR PRODUCING NITRILE
-
Paragraph 0080; 0090, (2021/02/05)
The present invention provides a method ...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego