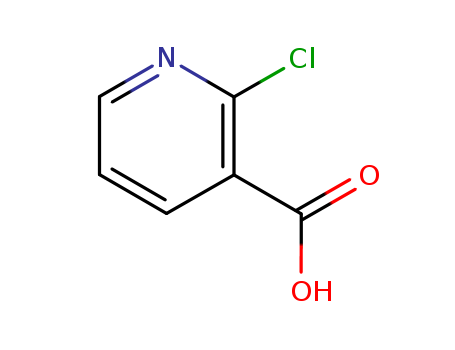
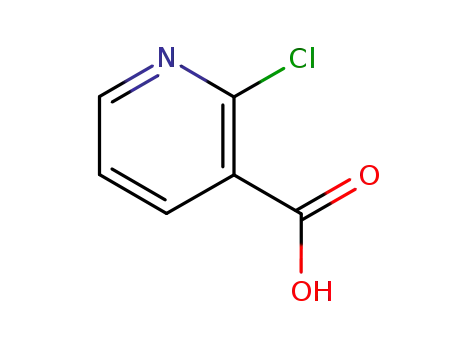
Quality Manufacturer Supply 2-Chloro Nicotinic Acid On Stock, Buy High Grade 2942-59-8
- Molecular Formula:C6H4ClNO2
- Molecular Weight:157.556
- Appearance/Colour:white to cream powder
- Vapor Pressure:0.000168mmHg at 25°C
- Melting Point:176-178 °C (dec.)(lit.)
- Boiling Point:316.8 °C at 760 mmHg
- PKA:2.07±0.25(Predicted)
- Flash Point:145.4 °C
- PSA:50.19000
- Density:1.47 g/cm3
- LogP:1.43320
2-Chloronicotinic acid(Cas 2942-59-8) Usage
|
Preparation
|
Chloronicotinic acid was synthesized from nicotinic acid by oxidation by H2O2 and then chlorinationby POCl3 and PCl5 in a yield of 87.5%.Synthesis of 2-chloronicotinic acid: Pour chlorine gas into the mixed solution of phosphorus oxychloride and phosphorus trichloride, control the temperature at about 60°C, until the remaining chlorine gas escapes, cool and add nicotinic acid N-oxide in batches, and heat the mixed solution. The reaction was carried out at 100-105°C for 1-1.5 h, and the reaction mixture was stirred for 30 min after the reaction mixture was transparent, and phosphorus oxychloride was removed under reduced pressure. The obtained residue was cooled to room temperature, and water was added to obtain the finished product of 2-chloronicotinic acid.
|
|
Used in Agrochemical Industries
|
2-Chloronicotinic acid finds extensive application in the agrochemical industries. It serves as a crucial intermediate for the synthesis of various herbicides and pharmaceutical drugs due to its versatility and importance as a building block. In agrochemicals, it contributes to the production of potent herbicides like diflufenican and nicosulfuron, known for their high biological activities, low toxicity, and broad spectrum.
|
|
Used in Pharmaceutical Industries
|
It is utilized in the synthesis of drugs such as nevirapine, mirtazapine, pranoprofen, and 2-aminonicotinic acids, highlighting its significance in medicinal chemistry.
|
|
Methods of production
|
The synthesis of 2-chloronicotinic acid involves several process routes, including methods using nicotinic acid nitrogen oxide, cyano-containing compounds, or 2-chloro-3-methyl-pyridine as raw materials, with the linear cyclization route being considered highly efficient due to its simplicity, minimal waste generation, and high yield.
Biocatalysis, particularly enzymatic reactions, offers an alternative and environmentally friendly approach for the synthesis of 2-chloronicotinic acid. Enzymes such as amidases play a significant role in catalyzing the hydrolysis of 2-chloronicotinamide to produce 2-chloronicotinic acid. Despite challenges in finding ideal catalysts, efforts in protein engineering aim to improve the catalytic properties of enzymes for efficient industrial production of 2-chloronicotinic acid through biocatalytic routes.
|
|
Application
|
2-Chloronicotinic acid is used in the synthesis of the anti-inflammatory and analgesic pralofen. It is an intermediate of the herbicides nicosulfuron and diflufenican. It is also used as pharmaceutical intermediates for the manufacture of mefenamic acid, niflumic acid, etc.
|
|
Consumer Uses
|
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment.
|
InChI:InChI=1/C6H4ClNO2/c7-5-4(6(9)10)2-1-3-8-5/h1-3H,(H,9,10)/p-1
2942-59-8 Relevant articles
-
Moehrle,Sieker
, p. 380,382 (1976)
-
Preparation method of 2-chloronicotinic acid
-
Paragraph 0029-0034, (2021/06/26)
The invention relates to a preparation m...
Combining photoredox catalysis and oxoammonium cations for the oxidation of aromatic alcohols to carboxylic acids
Nandi, Jyoti,Hutcheson, Ellen L.,Leadbeater, Nicholas E.
supporting information, (2020/12/25)
A methodology is reported for converting...
Preparation method of 2-chloronicotinic acid
-
Paragraph 0028-0045, (2020/06/05)
The invention belongs to the field of ch...
Preparation method of 2- novel chloronicotinic acid (by machine translation)
-
Paragraph 0045; 0046, (2020/02/14)
The method disclosed by the invention 2 ...
2942-59-8 Process route
-
- 2398-81-4
Nicotinic acid N-oxide
-
- 5326-23-8
6-Chloro-3-pyridinecarboxylic acid
-
- 2942-59-8
2-chloronicotinic acid
Conditions
| Conditions |
Yield |
|
With phosphorus pentachloride; trichlorophosphate;
|
|
-
- 18368-76-8
2 chloro-3-methylpyridine
-
- 2942-59-8
2-chloronicotinic acid
Conditions
| Conditions |
Yield |
|
With ozone; acetic acid; at 20 ℃; for 5h; Reagent/catalyst; Temperature;
|
98% |
|
With N-hydroxyphthalimide; oxygen; cobalt(III) acetylacetonate; In acetonitrile; at 80 ℃; for 18h; under 7500.75 Torr; Reagent/catalyst; Temperature; Autoclave;
|
94.1% |
|
With permanganate(VII) ion;
|
|
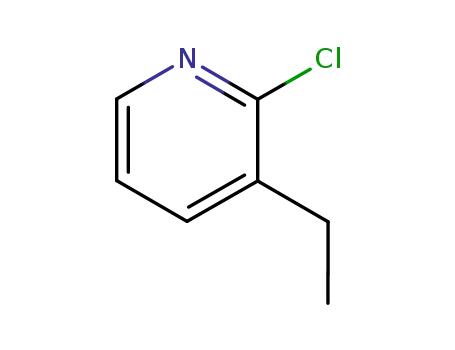
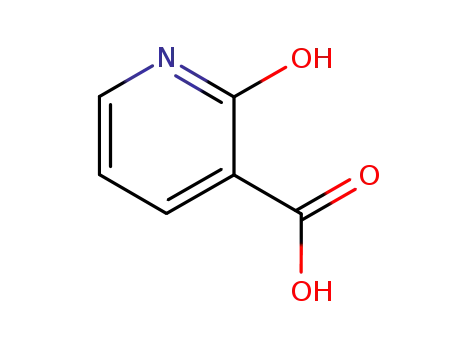
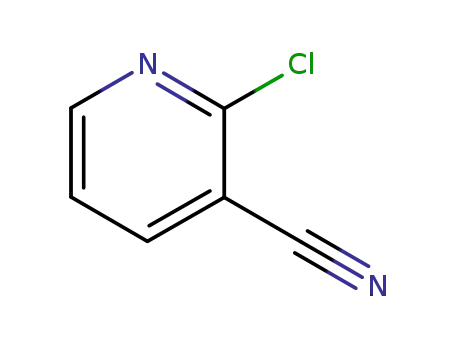
2942-59-8 Upstream products
-
18368-76-8
2 chloro-3-methylpyridine
-
96440-05-0
2-chloro-3-ethylpyridine
-
609-71-2
2-hydroxy-3-carboxypyridine
-
6602-54-6
2-chloro-3-pyridinecarbonitrile
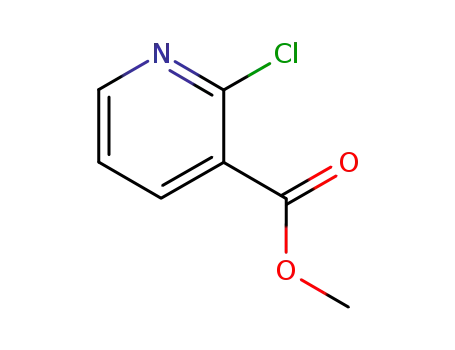
2942-59-8 Downstream products
-
40134-18-7
methyl 2-chloropyridine-3-carboxylate
-
609-71-2
2-hydroxy-3-carboxypyridine
-
99973-09-8
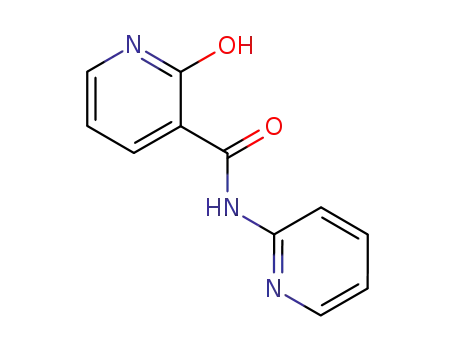
2-hydroxy-nicotinic acid-[2]pyridylamide
-
109255-91-6
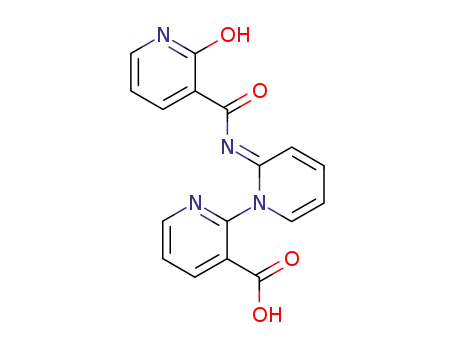
2-(2-hydroxy-nicotinoylimino)-2H-[1,2']bipyridyl-3'-carboxylic acid
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego