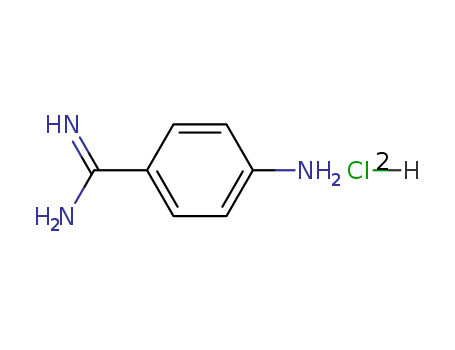
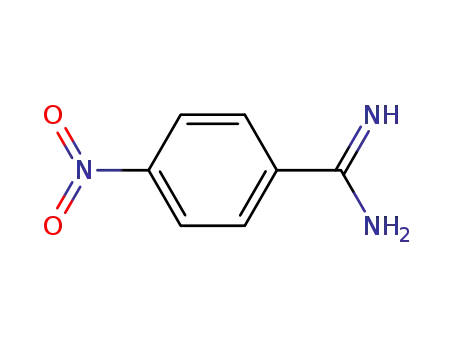
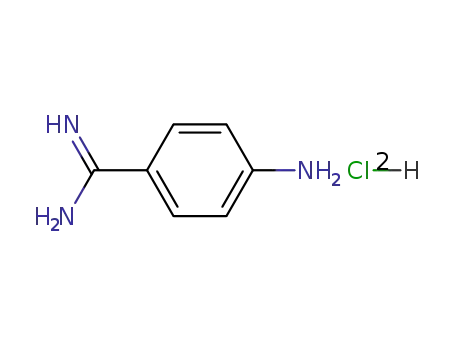
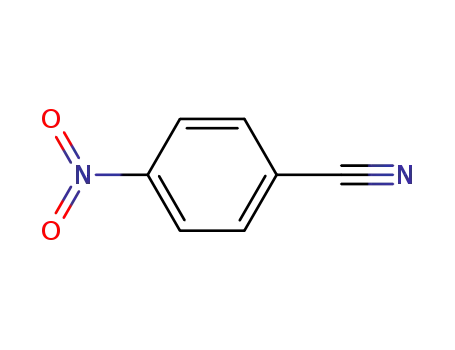
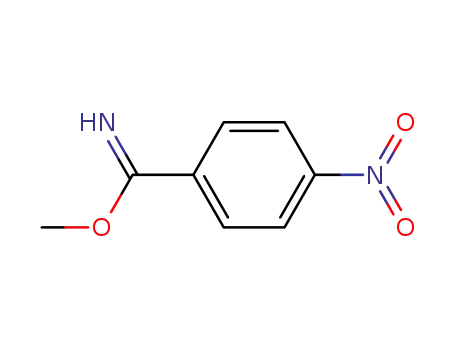
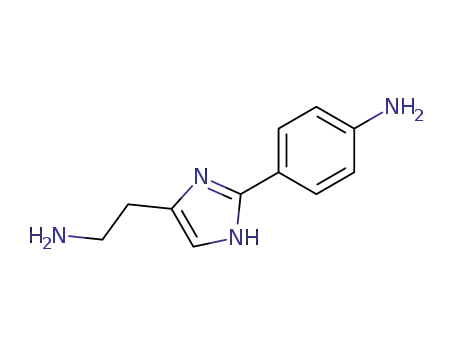
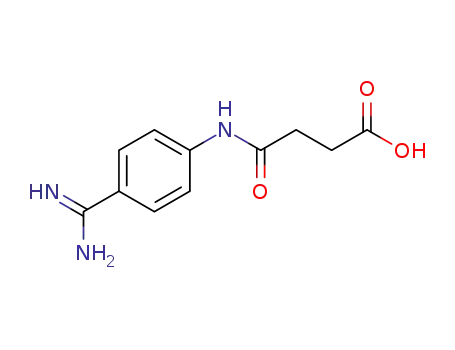
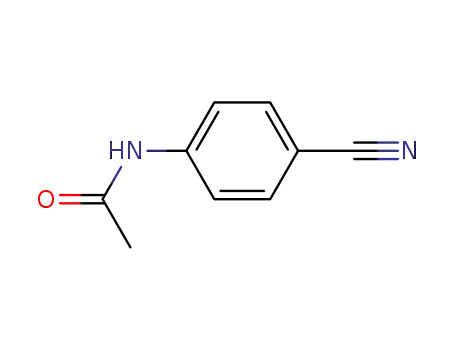Buy High Quality Quality Factory Supply 4-AminobenzamidineDihydrochloride 2498-50-2 with the Best Price
- Molecular Formula:C7H11Cl2N3
- Molecular Weight:208.09
- Appearance/Colour:white to yellowish fine crystalline powder
- Vapor Pressure:0.00216mmHg at 25°C
- Melting Point:>300 °C(lit.)
- Boiling Point:289.8 °C at 760 mmHg
- Flash Point:129 °C
- PSA:75.89000
- Density:1.26g/cm3
- LogP:3.53810
4-Aminobenzamidine dihydrochloride(Cas 2498-50-2) Usage
|
Preparation
|
4-Aminobenzamididine dihydrochloride is obtained by cyanation, addition, amination and reduction of 4-nitrobenzoic acid.
|
|
Application
|
4-Aminobenzamidine dihydrochloride can be used to synthesize:Orally active fibrinogen receptor antagonists based on benzamidines.Benzamidine derivatives that are selective and potent serine protease inhibitors.Novel pyrrolo [3,2-c] quinolines that are structural analogs of topoisomerase inhibitors such as coralyne and fagaronine.Synthesis of a selective inhibitor of PRMT1 (SKLB-639)
|
InChI:InChI=1/C7H9N3/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H3,9,10)/p+1
2498-50-2 Relevant articles
Novel preparation method of p-aminobenzamidine hydrochloride
-
Paragraph 0032; 0033; 0035; 0037; 0039; 0041; 0043; 0045, (2018/11/22)
The invention discloses a novel preparat...
Design, Synthesis, and Testing of Potent, Selective Hepsin Inhibitors via Application of an Automated Closed-Loop Optimization Platform
Pant, Shishir M.,Mukonoweshuro, Amanda,Desai, Bimbisar,Ramjee, Manoj K.,Selway, Christopher N.,Tarver, Gary J.,Wright, Adrian G.,Birchall, Kristian,Chapman, Timothy M.,Tervonen, Topi A.,Klefstr?m, Juha
supporting information, p. 4335 - 4347 (2018/05/14)
Hepsin is a membrane-anchored serine pro...
A to prepare to aminobenzoic amidine hydrochloride method
-
, (2017/02/02)
The invention relates to the technical f...
Design, synthesis and structure-activity relationships of novel diaryl urea derivatives as potential EGFR inhibitors
Jiang, Nan,Bu, Yanxin,Wang, Yu,Nie, Minhua,Zhang, Dajun,Zhai, Xin
, (2016/12/03)
Two novel series of diaryl urea derivati...
2498-50-2 Process route
-
- 25412-75-3
4-nitrobenzamidine
-
- 2498-50-2
4-aminobenzamidine dihydrochloride
Conditions
| Conditions |
Yield |
|
4-nitrobenzamidine; With iron; acetic acid; In ethanol; water; at 60 - 80 ℃; for 4h;
With hydrogenchloride; In ethanol; water; Reagent/catalyst;
|
94.7% |
-
- 35704-19-9
N-(4-cyanophenyl)acetamide
-
- 2498-50-2
4-aminobenzamidine dihydrochloride
Conditions
| Conditions |
Yield |
|
N-(4-cyanophenyl)acetamide; With sodium amide; In dimethyl sulfoxide; at 20 - 80 ℃; for 1.5h; Large scale;
With hydrogenchloride; In ethanol; dimethyl sulfoxide; at 0 ℃; for 1.5h; pH=3 - 4; Solvent; Reagent/catalyst; Temperature; Large scale;
|
97.8% |
2498-50-2 Upstream products
2498-50-2 Downstream products
-
133118-43-1
2-<2-(4-aminophenyl)-4-imidazolyl>ethanamine
-
153982-08-2
4-<<4-(aminoiminomethyl)phenyl>amino>-4-oxobutanoic acid
-
147291-70-1
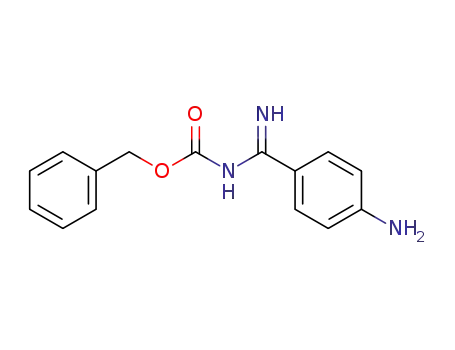
4-[N-(benzyloxycarbonyl)-aminoiminomethyl]aniline
-
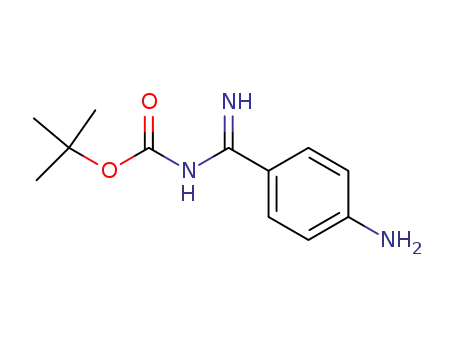
147291-56-3
tert-butyl [(4-aminophenyl)(imino)methyl]carbamate
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego