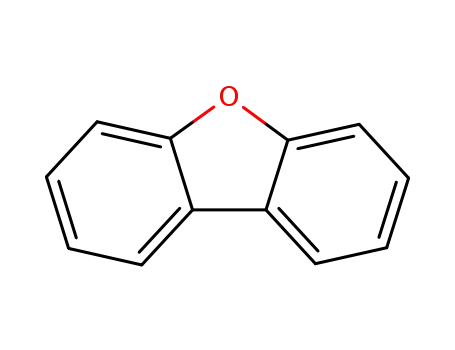
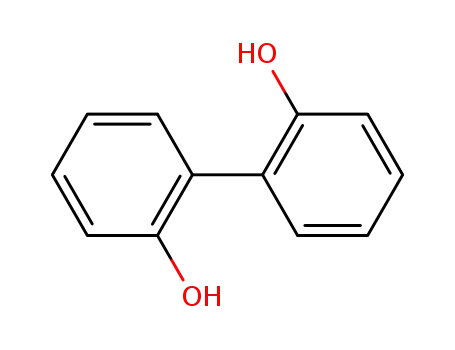
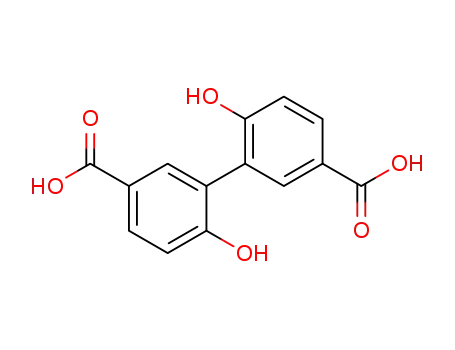
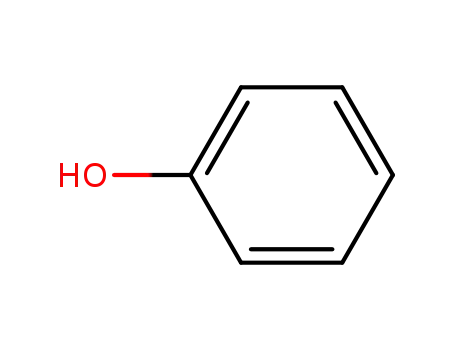
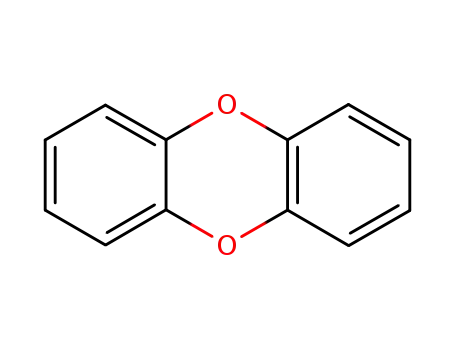
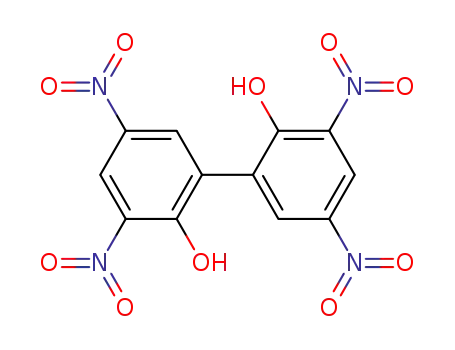
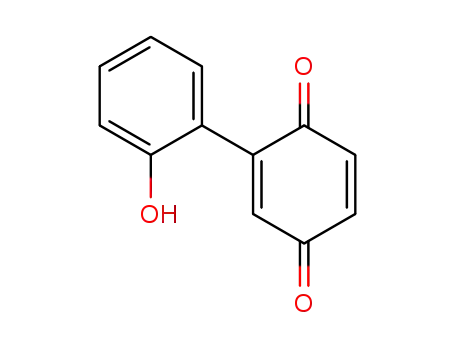
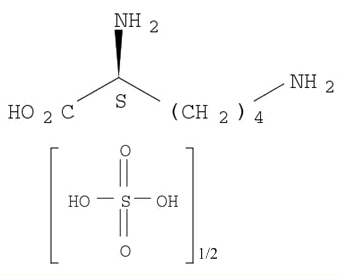
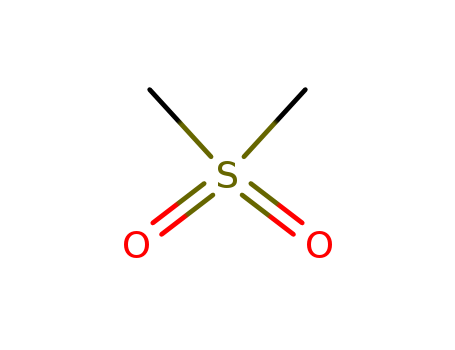
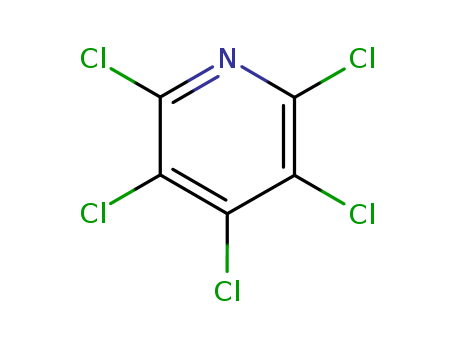
Products, rates, and mechanism of the gas-phase condensation of phenoxy radicals between 500-840 K
Wiater, Izabela,Born, Jan G. P.,Louw, Robert
, p. 921 - 928 (2000)
Phenols are demonstrated precursors of '...
THE ROLE OF STEREOELECTRONIC FACTORS IN THE OXIDATION OF PHENOLS
Armstrong, David R.,Breckenridge, Robin J.,Cameron, Colin,Nonhebel, Derek C.,Pauson, Peter L.,Perkins, Peter G.
, p. 1071 - 1074 (1983)
The oxidative coupling of both 3,5-dimet...
Synthesis of the spirocyclic cyclohexadienone ring system of the schiarisanrins.
Coleman,Guernon,Roland
, p. 277 - 280 (2000)
[structure: see text] Studies on the syn...
-
Britton,zit. bei Gilman,Swiss,Cheney
, p. 1963,1964 (1940)
-
CuO grafted triazine functionalized covalent organic framework as an efficient catalyst for C-C homo coupling reaction
Das, Sabuj Kanti,Krishna Chandra, Bijan,Molla, Rostam A.,Sengupta, Manideepa,Islam, Sk. Manirul,Majee, Adinath,Bhaumik, Asim
, (2020)
Designing of low cost catalytic system f...
Photochemistry of Dibenzo-1,4-dioxin: Formation of 2,2'-Biphenylquinone as an Observable Intermediate
Guan, Bing,Wan, Peter
, p. 409 - 410 (1993)
Photolysis of dibenzo-1,4-dioxin 1, whic...
Photochemical rearrangement of chlorinated dibenzo-p-dioxins. Regioselective carbon-oxygen bond homolysis from the singlet excited state, and carbon-chlorine bond homolysis from the triplet excited state
Kobayashi, Takanori,Shimada, Jun-Ichi,Kitahara, Chieko,Haga, Naoki
, p. 348 - 349 (2006)
UV light irradiation of 1-chloro-, 2-chl...
Small Molecule NF-κB Inhibitors as Immune Potentiators for Enhancement of Vaccine Adjuvants
Moser, Brittany A.,Escalante-Buendia, Yoseline,Steinhardt, Rachel C.,Rosenberger, Matthew G.,Cassaidy, Britteny J.,Naorem, Nihesh,Chon, Alfred C.,Nguyen, Minh H.,Tran, Ngoctran T.,Esser-Kahn, Aaron P.
, (2020)
Adjuvants are added to vaccines to enhan...
Phenol conversion and dimeric intermediates in horseradish peroxidase-catalyzed phenol removal from water
Jian Yu,Taylor,Huixian Zou,Biswas,Bewtra
, p. 2154 - 2160 (1994)
Phenol was removed from water by horsera...
SECOND-ORDER COMBINATION REACTION OF PHENOXYL RADICALS
Ye, Mingyu,Schuler, Robert H.
, p. 1898 - 1902 (1989)
Phenoxy radicals, when produced pulse ra...
Dendrimer-encapsulated Pd nanoparticles as aqueous, room-temperature catalysts for the Stille reaction
Garcia-Martinez, Joaquin C.,Lezutekong, Raphael,Crooks, Richard M.
, p. 5097 - 5103 (2005)
We report that dendrimer-encapsulated Pd...
Ir-Catalyzed Ligand-Free Directed C-H Borylation of Arenes and Pharmaceuticals: Detailed Mechanistic Understanding
Mahamudul Hassan, Mirja Md,Mondal, Biplab,Singh, Sukriti,Haldar, Chabush,Chaturvedi, Jagriti,Bisht, Ranjana,Sunoj, Raghavan B.,Chattopadhyay, Buddhadeb
supporting information, p. 4360 - 4375 (2022/03/16)
An efficient method for Ir-catalyzed lig...
A mild and practical method for deprotection of aryl methyl/benzyl/allyl ethers with HPPh2andtBuOK
Pan, Wenjing,Li, Chenchen,Zhu, Haoyin,Li, Fangfang,Li, Tao,Zhao, Wanxiang
, p. 7633 - 7640 (2021/09/22)
A general method for the demethylation, ...
Conversion method of dibenzo[c,e][1,2]oxathiane-6-oxide compound
-
Paragraph 0073; 0075; 0101-0106, (2020/02/04)
The invention relates to the technical f...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego