|
Indications
|
Doxycycline (Vibramycin, Monodox) has similar absorption and durationof- activity characteristics. Its effectiveness in acne approaches that of minocycline, when used in the same fashion with similar dosages. Early data suggests that subantimicrobial doses of doxycycline, 20 mg (Periostat), may play a therapeutic role in acne by reducing inflammation through anticollagenolytic, antimatrix-degrading metalloproteinase, and cytokine downregulating properties.
|
|
Manufacturing Process
|
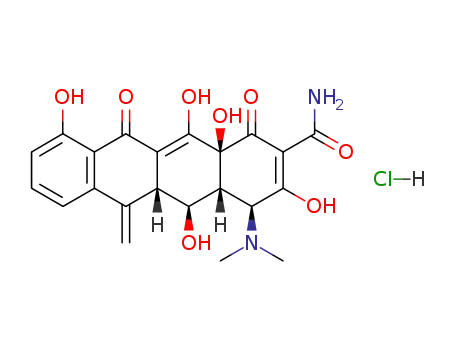
Hydrogen was introduced into a standard hydrogenation vessel containing 10 grams 6-deoxy-6-demethyl-6-methylene-5-oxytetracycline hydrochloride(methacycline), 150 ml methanol and 5 grams 5% rhodium on carbon. The pressure was maintained at 50 psi while agitating at room temperature for 24 hours. The catalyst was then filtered off, the cake washed with methanol and the combined filtrates were evaporated to dryness. The dry solids were slurried in ether, filtered and the cake dried. The resulting solids exhibited a bioactivity of 1,345 units per mg versus K. pneumoniae.Water (35 ml) was employed to dissolve 8.5 grams of the above product and the pH was adjusted to 6.0 with triethylamine, sufficient dimethyl formamide being added to maintain the solids in solution. Cellulose powder (2 kg) was slurried in water-saturated ethyl acetate and packed into a tower of about 3? inches diameter, to a height of 3 ft. The product solution was then chromatographed over this column, developing with about 12 liters water�saturated ethyl acetate. The first product fraction to come from the tower yielded 1.85 grams 6-epi-6-deoxy-5-oxytetracycline. The next fraction contained 2.0 grams of 6-deoxy-6-demethyl-6-methylene-5-oxytetracycline. The third fraction yielded 0.8 grams 6-deoxy-5-oxytetracycline.
|
|
Therapeutic Function
|
Antibiotic
|
|
Antimicrobial activity
|
It is active against some tetracycline-resistant Staph. aureus and is more active than other tetracyclines against Str. pyogenes, enterococci and Nocardia spp. Mor. catarrhalis (MIC 0.5 mg/L), Legionella pneumophila and most strains of Ureaplasma urealyticum (MIC 0.5 mg/L) are susceptible.
|
|
Pharmaceutical Applications
|
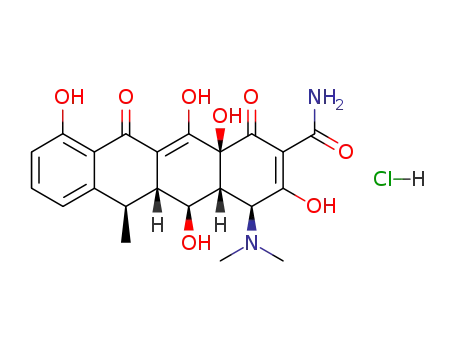
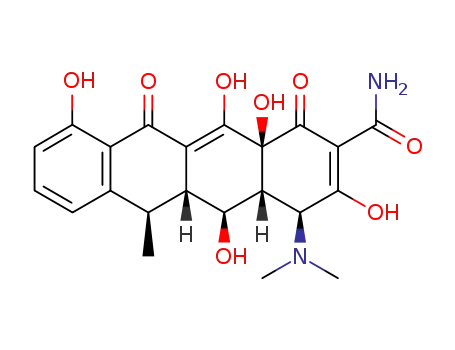
6-Deoxy-5β-hydroxytetracycline. A semisynthetic product supplied as the hyclate, calcium salt or the hydrochloride for oral and intravenous administration.
|
|
Pharmacokinetics
|
Oral absorption: 90% Cmax 100–200 mg oral: 1.7–5.7 mg/L after 2–3.5 h 100 mg intravenous infusion (1 h): 2.5 mg/L end infusion Plasma half-life:18 h Volume of distribution: 0.9–1.8 L/kg Plasma protein binding: 90% Absorption Doxycycline is rapidly absorbed from the upper gastrointestinal tract and absorption appears to be linearly related to the administered dose. Food, especially dairy products, reduces peak serum concentrations by 20%. Alcohol also delays absorption. As with other tetracyclines, divalent and trivalent cations, as in antacids and ferrous sulfate, form chelates which reduce absorption. Distribution The greater lipophilicity of doxycycline is responsible for its widespread tissue distribution. Concentrations in liver, biliary system, kidneys and the digestive tract are approximately twice those in plasma. Within the respiratory tract, it achieves concentrations of 2.3–6.7 mg/kg in tonsils and 2.3–7.5 mg/kg in maxillary sinus mucosa. In bronchial secretions concentrations are about 20% of plasma levels, increasing to 25–35% in the presence of pleurisy. Gallbladder concentrations are approximately 75% those of plasma, and prostate concentrations are 60–100%. It penetrates well into the aqueous humor. CSF concentrations range from 11% to 56% of plasma levels and are not affected by inflammation. In the elderly, tissue concentrations are 50–100% higher than in young adults. The half-life remains unaltered and one explanation is reduced fecal elimination. Metabolism and excretion Doxycycline is largely excreted unchanged. Around 35% is eliminated through the kidneys and the remainder through the digestive tract. Renal clearance ranges from 1.8 to 2.1 L/h, and is largely via glomerular filtration, with approximately 70% tubular reabsorption. Alkalinization enhances renal clearance. Fecal elimination partly reflects biliary excretion but also includes diffusion across the intestinal wall. Provided the drug is not chelated, reabsorption occurs with enterohepatic recycling. The elimination half-life is long (15–25 h). The half-life and the area under the concentration–time curve (AUC) are little altered in renal insufficiency, with no evidence of accumulation after repeat dosing, even in anuric patients, evidently as a result of increased clearance through the liver or gastrointestinal tract, since biliary and fecal concentrations increase in renal failure. Although the plasma elimination half-life is unchanged, the drug appears to accumulate in tissues with increasing renal failure, and it has been suggested that less drug is bound to plasma protein and red cells through competition with other metabolites, which in turn increases hepatic elimination. Pharmacokinetics are unaltered by hemodialysis or peritoneal dialysis. Clearance is decreased by about half in patients with type IIa and type IV hyperlipidemia. The plasma elimination half-life is shortened by various antiepileptic agents including phenytoin, barbiturates and carbamazepine, presumably as a result of liver enzyme induction, although there is also evidence for some interference with the protein binding of doxycycline.
|
|
Side effects
|
Untoward reactions are generally those typical of the group but gastrointestinal side effects are less common than with other tetracyclines due to the lower total dosage and the ability to administer the drug with meals. Esophageal ulceration as a result of capsule impaction has been reported. Dental and bone deposition appear to be less common than with other tetracycline derivatives. Other adverse phenomena include occasional vestibular toxicity. Hypersensitivity reactions include photosensitivity and eosinophilia, but rarely anaphylaxis. In common with demeclocycline and chlortetracycline it may be a more powerful sensitizer than other tetracyclines. It is contraindicated in patients with acute porphyria because it has been demonstrated to be porphyrinogenic in animals.
|
|
Synthesis
|
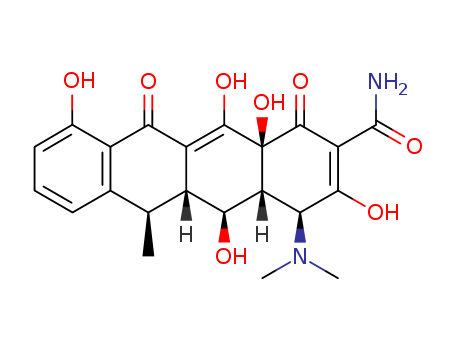
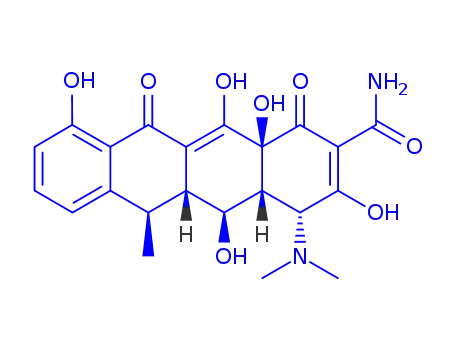
Doxycycline, 4-dimethylamino-1,4,4a,5,5a,6,11,12a-oxtahydro-3,5,10,12, 12a-pentahydroxy-6-methyl-1,11-dioxo-2,naphthacencarboxamide (32.3.7), is an isomer of tetracycline that differs only in the placement of one hydroxyl group. Doxycycline can be formally viewed as the result of transferring the C6 hydroxyl group of tetracycline to C5. Doxycycline is synthesized in two different ways from oxytetracycline (32.3.2). One of the ways suggests dehydrating oxytetracycline at C6 by reducing the tertiary hydroxyl group with hydrogen using a rhodium on carbon catalyst. The second way is analogous to that of giving methacycline, which suggests an oxidation stage of the homoallyl system, except that N-chlorosuccinimide is used as the oxidant, which results in the formation of a naphthacentetrahydrofuran derivative (32.3.8), and which upon being reacted with hydrofluoric acid breaks apart to form an 11a-chloro- 6-exomethylene derivative (32.3.9). Reductive dechlorination of this product using sodium thiosulfate forms the intermediate methacycline (32.3.6), and thiophenol is joined to the methyl group that carry out radical reactions, forming the derivative (32.3.10). This product is reduced by hydrogen over a Raney nickel catalyst, during which reductive desulfurization takes places, giving doxycycline.
|
|
Drug interactions
|
Potentially hazardous interactions with other drugsAnticoagulants: possibly enhanced anticoagulant effect of coumarins and phenindione.Ciclosporin: possibly increases plasma-ciclosporin concentration.Oestrogens: possibly reduced contraceptive effects of oestrogens (risk probably small) Retinoids: possible increased risk of benign intracranial hypertension - avoid.
|
|
Metabolism
|
Doxycycline is well absorbed on oral administration (90–100% when fasting; reduced by 20% by co-consumption with food or milk), has a half-life permitting once-a-day dosing for mild infections, and is excreted partly in the feces and partly in the urine.
|
|
Dosage forms
|
50 mg b.i.d. to q.i.d.; 100 mg q.d. to b.i.d. Recent evidence suggest that sub-antimicrobial dose of 20 mg b.i.d. is also effective. No dosage adjustments needed for renal impairment.
|
|
Definition
|
ChEBI: Tetracycline in which the 5beta-hydrogen is replaced by a hydroxy group, while the 6alpha-hydroxy group is replaced by hydrogen. A semi-synthetic tetracycline antibiotic, it is used to inhibit bacterial protein synthesis a d treat non-gonococcal urethritis and cervicitis, exacerbations of bronchitis in patients with chronic obstructive pulmonary disease (COPD), and adult periodontitis.
|
|
General Description
|
A more recent addition to the tetracycline group of antibioticsavailable for antibacterial therapy is doxycycline,α-6-deoxy-5-oxytetracycline (Vibramycin), first reportedby Stephens et al. in 1958. It was obtained first in smallyields by a chemical transformation of oxytetracycline, butit is now produced by catalytic hydrogenation of methacyclineor by reduction of a benzyl mercaptan derivative ofmethacycline with Raney nickel. The latter processproduces a nearly pure form of the 6α-methyl epimer. The6α-methyl epimer is more than 3 times as active as itsβ-epimer.169 Apparently, the difference in orientation of themethyl groups, which slightly affects the shapes of the molecules,causes a substantial difference in biological effect. Also, absence of the 6-hydroxyl group produces acompound that is very stable in acids and bases and that hasa long biological half-life. In addition, it is absorbed verywell from the GI tract, thus allowing a smaller dose to be administered.High tissue levels are obtained with it, and unlikeother tetracyclines, doxycycline apparently does not accumulatein patients with impaired renal function.Therefore, it is preferred for uremic patients with infectionsoutside the urinary tract. Its low renal clearance may limit itseffectiveness, however, in urinary tract infections.Doxycycline is available as a hydrate salt, a hydrochloridesalt solvated as the hemiethanolate hemihydrate, and amonohydrate. The hydrate form is sparingly soluble in waterand is used in a capsule; the monohydrate is water insolubleand is used for aqueous suspensions, which are stable for upto 2 weeks when kept in a cool place.
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego