|
Vitamin D
|
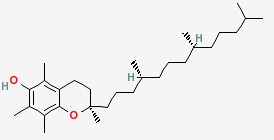
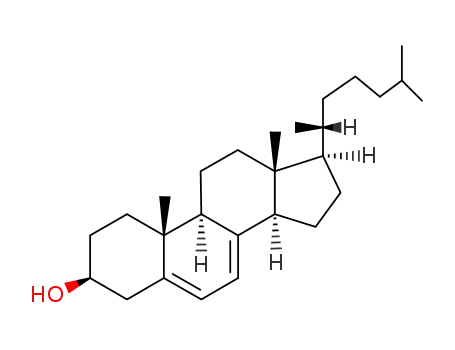
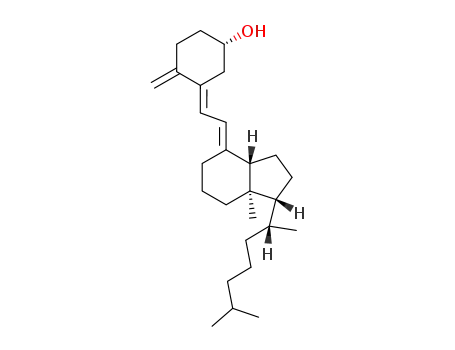
Vitamin D is steroid derivatives. It is the general term for the compounds that have similar structures and have the effect of normal skeletal growth and maintenance. At present, there are at least more than ten kinds of sterols that have vitamin D activity. But there are only two important matters, which are known as pro-vitamin D. One is ergosterol that exists in vegetable oil or yeast, which can generate vitamin D2 when irradiated by sunlight or ultraviolet radiation. It is also known as cholecalciferol or ergocalciferol. Another is 7-dehydrochlesterol that exists in the skin. It will become vitamin D3 after irradiated by the light. It is also called cholecalciferol. D2 and D3 have similar structures. They are B ring derivatives of precursors of the sterol. The difference is that the side chain of vitamin D2 has a methyl group and one double bond. Vitamin D2 and D3 are all colorless crystals. The melting point of D2 and D3 respectively are 115~118℃ and 84~85℃. They are insoluble in water, and soluble in ethanol and other organic solvents. Their solubility in vegetable oil are small. Crystalline vitamin D should be stored in dark to light and oxygen at low temperature. Vitamin D3 is more stable than D2. It is not easy to be oxidized. Vitamin D has heat stability in the near neutral solution. It will be rapidly destroyed in the acidic solution, and the rate of destruction depends on strength and temperature of the acid. However, it is stable in alkaline solution. It can tolerate alkali treatment even under high temperature conditions. In the industrial synthesis products, vitamin D3 is more. Vitamin D3 is sensitive to light and can be damaged by minerals and oxidation. But vitamin D3 has good stability after esterification and coated by gelatin, sugar and starch. The activity of vitamin D3 is also represented by International Units (IU). The amount of IU crystals vitamin D3 is 0.025μg. The active ingredient content of the industrial synthesis of vitamin D3 additive is more of 500,000 IU/g or 200 000 IU/g. For vitamin additives products, there are also additives that contain vitamin A and vitamin D3. The activity is usually 1g additive containing 500,000 IU of vitamin A and 100,000 IU of vitamin D3. The two has no antagonistic effect. And their production and use are very convenient. Vitamin D can increase the absorption and metabolism of calcium and phosphorus, prevent swine rickets, and promote the development of teeth and bones. Vitamin D dosage of pig feed is 125~200IU/kg. The amount for piglets is higher than growing pig. The amount of boar and growing pig are similar.
|
|
Physiological function
|
Under UV irradiation vegetative ergosterol and animal 7-dehydrogenation are absorbed and convert into vitamin D2 and vitamin D3. The two vitamin D must produc further chemical changes before used by the body. 7-dehydrocholesterol first form into 25-hydroxy derivative in the liver, then generate active ingredient 1,25-dihydroxyvitamin cholecalciferol [1,25 (OH) 2D5] by hydroxylation. The main function of vitamin D is to promote intestinal absorption of calcium and phosphorus, and regulate metabolism of calcium and phosphorus in order to maintain the body's calcium and phosphorus balance. Calcium absorbed from intestinal canal can be transported through the blood circulation from the liver and kidneys to the bone and other calcified tissues, and excret from the bone to the kidneys. This transport mechanism is controlled by vitamin D. When the concentration of calcium, phosphorus in the body decreases, vitamin D can dissociate calcium from the bone. In addition, vitamin D can also control kidney tubules to reabsorb calcium and phosphorus. Vitamin D2 and vitamin D3 has the same function on ammals cows and pigs. But vitamin D3 for poultry (birds) is ten times stronger activity than vitamin D2. Vitamin D deficiency can reduce the intestinal absorption of calcium, and cause the decomposition of bone calcium and phosphorus. Young livestock will appear osteomalacia. Adult animals are easy to appear osteoporosis. Vitamin D deficiency can also lead to animal sternum and spine deformation and layers laying soft-shell eggs. Because vitamin D controls the absorption of calcium and phosphorus, too much vitamin D in dietary can cause hypercalcemia, make excess calcium deposit in the heart, blood vessels, joints, pericardium or intestinal wall, and finally lead to heart failure, joint stiffness or bowel Road disorders. Sunbathing is the most economical source of vitamin D. ergosterol or 7-dehydrogenation cholesterol in the feed will turn into vitamin D by ultraviolet radiation inside an animal for the utilization of carcass. Hay, corn leaves yeast, barley, oats, wheat after insolation and yeast after ultraviolet treatment are better sources of vitamin D. Animal products can be directly provide vitamin D to livestock, such as eggs, milk. The content of vitamin D in cod liver oil is very high. Diets need to be added vitamin D for reared animals and insufficient sunlight animal. The above information is edited by the lookchem of Ge Qian.
|
|
Content analysis
|
Same with “vitamin D2 (01043)”. But all ergocalciferol is replaced by cholecalciferol.
|
|
Toxicity
|
LD50 oral in rat: 42mg/kg
|
|
Maximum level
|
GB 14880-94: same with “01043, vitamin D2” FDA, §18. 1950(2000): same with “01043, vitamin D2”
|
|
Chemical property
|
White columnar crystals or crystalline powder, odorless and tasteless. Melting point 84~88℃, specific rotation αD20 =+105°~+112°. Easily soluble in chloroform, soluble in alcohol, ether, cyclohexane and acetone, slightly soluble in vegetable oil, insoluble in water. Good heat resistance, but unstable for light. Easily oxidized in air. Rats by oral LD1042mg/kg.
|
|
Production method
|
1. 7-dehydrocholesterol is dissolved in ethanol, and then treated with ultraviolet light to open the ring. The reaction mixture is concentrated, frozen and filtered. The filtrate is concentrated under reduced pressure nitrogen to dryness to give a crude liquid vitamin D3. Then it is refined to get vitamin D3. 2. Vitamin D3 naturally exists in the liver, egg yolk and milk. The production method in the industry starts from vegetable oil or yeast to extract 7-dehydrocholesterol that is not absorbed by the human body. Then it is dissolved in chloroform or cyclohexane. And then it converts to vitamin D3 by the ultraviolet radiation in the quartz glass flask. 3. Vitamin D3 naturally exists in the liver, egg yolk and milk. The production method in the industry starts from vegetable oil or yeast to extract 7-dehydrocholesterol that is not absorbed by the human body. Then it is dissolved in chloroform or cyclohexane. And then it converts to vitamin D3 by the ultraviolet radiation in the quartz glass flask.
|
|
Indications
|
Vitamin D is the collective term for a group of compounds formed by the action of ultraviolet irradiation on sterols. Cholecalciferol (vitamin D3) and calciferol (vitamin D2) are formed by irradiation of the provitamins 7- dehydrocholesterol and ergosterol, respectively. The conversion to vitamin D3 occurs in the skin. The liver is the principal storage site for vitamin D, and it is here that the vitamin is hydroxylated to form 25-hydroxyvitamin D. Additional hydroxylation to form 1,25-dihydroxyvitamin D occurs in the kidney in response to the need for calcium and phosphate
|
|
Manufacturing Process
|
5 g of 7-dehydrocholesteryl acetate (prepared by W.R. Ness, R. S. Kostic and Mosetting, J. Am. Chem. Soc. 78, 436, 1956) were dissolved in 500 ml of n�hexane. This solution was irradiated with ultraviolet ray by recyclicly passing it through a quartz apparatus surrounding 450 w high pressure mercury vapor lamps for 80 minutes. After irradiation and then the distillating off of n�hexane the solution was added with 50 ml of ethanol and the ethanolic solution was left to stand overnight at the temperature of -20°C. The formed crystals were filtered off from ethanolic solution and filtrate was heated at the temperature 78°C for 4 hours. After cooling of filtrate, the cooled filtrate was added with 4 ml of ethanolic solution containing 0.7 g of potassium hydroxide to effect a reaction at the temperature of 20°C and under nitrogen for 60 minutes. The reaction product was added with 0.7 ml glacial acetic acid and then ethanol was distilled off under reduced pressure from the reaction product. The obtained residue was extracted with 50 ml of n-hexane and extract was washed with water and n-hexane was distilled off from extract to obtain 2.5 g of yellow oily matter containing vitamin D3. The content of vitamin D3 in yellow oily matter was 40.2% by weight.
|
|
Therapeutic Function
|
Vitamin, Antirachitic
|
|
Biosynthesis
|
The primary supply of vitamin D3 in humans is not obtained from the diet but rather is derived from the ultraviolet photoconversion of 7-dehydrocholesterol to vitamin D3 in skin. Thus, vitamin D3 synthesis varies with the seasons. D3 is a prohormone and requires further metabolic conversion to exert biological activity in its target organs. The liver and the kidney are the major sites of metabolic activation of this endogenous sterol hormone. The initial transformation of D3 occurs in the liver and is catalyzed by the enzyme 25-OH-D3-hydroxylase to form 25-(OH)D3; this is the primary circulating form of D3. Circulating 25-(OH)D3 is then converted by the kidney to the most active form of D3, 1,25-(OH)2D3, by the 1-(OH)-D3-hydroxylase enzyme. Blood concentrations of 1,25-(OH)2D3 are approximately one fivehundredth of those of 25-(OH)D3. 1, 25-(OH)2D3 is converted to the metabolite 24R,25-(OH)2D3, which is capable of suppressing parathyroid secretion. In addition to the endogenous metabolites, some exogenous sterols possess biological activity similar to that of D3. Ergocalciferol (vitamin D2) is derived from the plant sterol ergosterol and may act as a substrate for both the 25-hydroxylase and the 1-hydroxylase enzyme systems of the liver and kidney to form 25-(OH)D2 and 1,25-(OH)2 D2, respectively. Ergocalciferol (vitamin D2) is the form used in commercial vitamins and supplemented dairy products. Dihydrotachysterol, another sterol that is used as a therapeutic agent, also functions as a substrate for the hydroxylase enzymes in the liver and kidney.
|
|
Health Hazard
|
SYMPTOMS: Symptoms of exposure to Vitamin D3 may include weakness, fatigue, lassitude headache, nausea, vomiting, diarrhea, polyuria, polydipsia, nocturia, decrease urinary concentrating ability, proteinuria, tissue calcification, hypertension and osteoporosis.
|
|
Fire Hazard
|
Flash point data for Vitamin D3 are not available. Vitamin D3 is probably combustible.
|
|
Trade name
|
DELSTEROL?; DEPARAL?; D3- VIGANTOL?; QUINTOX?; RAMPAGE?; RICKETON?; TRIVITAN?; VIGORSAN?; VITINC DAN-DEE-3?
|
|
Biochem/physiol Actions
|
Vitamin D acts through a receptor that is a member of the ligand-dependent transcription factor superfamily. Modulates the proliferation and differentiation of both normal and cancer cells. Has antiproliferative and antimetastatic effects on breast, colon, and prostate cancer cells. Activated vitamin D receptors in intestine and bone maintain calcium absorbance and homeostasis.
|
|
Mechanism of action
|
1, 25-(OH)2D3 exerts its influence within target tissues through high-affinity sterol-specific intracellular receptor proteins.The D3 receptor, similar to steroid receptor systems, translocates the hormone from the cell cytoplasm to the nucleus, where biological response is initiated via transcription and translation.
|
|
Side effects
|
The hypercalcemia resulting from hypervitaminosis D is responsible for toxic symptoms such as muscle weakness, bone pain, anorexia, ectopic calcification, hypertension, and cardiac arrhythmias. Toxicity in infants can result in mental and physical retardation, renal failure, and death.
|
|
Safety Profile
|
Poison by ingestion. An experimental teratogen. When heated to decomposition it emits acrid smoke and irritating fumes.
|
|
Potential Exposure
|
Sterol rodenticide used in bait for vermin control. Vitamin D is a steroid hormone that has an important role in regulating body levels of calcium and phosphorus, and in mineralization of bone. Not approved for use in EU countries
|
|
Drug interactions
|
Potentially hazardous interactions with other drugs Antiepileptics: the effects of vitamin D may be reduced in patients taking barbiturates or anticonvulsants. Diuretics: increased risk of hypercalcaemia with thiazides. Sevelamer: absorption may be impaired by sevelamer.
|
|
Metabolic pathway
|
Cholecalciferol (vitamin D3) is the mammalian form of vitamin D. It is normally produced in the skin by the action of UV light on its precursor, 7- dehydrocholesterol. Essential amounts of the vitamin are obtained thus or from dietary sources such as fish oils. The active form of the vitamin is 1,25-dihydroxy-cholecalciferol. Its formation occurs in two stages: 25- hydroxylation in the liver, followed by 1-hydroxylation in the kidney (see Engstrom and Koszewski, 1989 and references cited therein).
|
|
Metabolism
|
Within the liver, cholecalciferol is hydroxylated to calcidiol (25-hydroxycholecalciferol) by the enzyme 25-hydroxylase. Within the kidney, calcidiol serves as a substrate for 1-alpha-hydroxylase, yielding calcitriol (1,25-dihydroxycholecalciferol), the biologically active form of vitamin D3.Cholecalciferol and its metabolites are excreted mainly in the bile and faeces.
|
|
Purification Methods
|
It is converted into its 3,5-dinitrobenzoyl ester and crystallised repeatedly from acetone. The ester is then saponified and the free vitamin is isolated. [Laughland & Phillips Anal Chem 28 817 1956, Beilstein 6 III 2811, 6 IV 4149.]
|
|
Degradation
|
It is unstable in light and air and in acidic media. It is inactivated within a few days under normal exposure conditions. This is due to oxidation and fragmentation of the triene functionality.
|
|
Toxicity evaluation
|
Another steroidal rodenticide is cholecalciferol, which is in fact the naturally occurring vitamin D3. This compound is an essential factor for vertebrates but in large doses causes hypercalcemia, resulting in calcification and degeneration of various soft tissues, ultimately leading to death. In baits, cholecalciferol may be combined with other, usually anticoagulant, rodenticides. The main natural source of cholecalciferol is fish liver oil, but it is manufactured from ergosterol.
|
|
Incompatibilities
|
Sensitive to air, light, and moisture. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
|
|
Waste Disposal
|
Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations.
|
|
Definition and FDA-Approved Indications
|
Cholecalciferol, also known as vitamin D3, is a dietary supplement prescribed for individuals with vitamin D insufficiency or deficiency. It is FDA-approved for use as a dietary supplement.
|
|
Mechanism of Action
|
Cholecalciferol is inactive until converted into 25-hydroxyvitamin D3 (25(OH)D3) in the liver by the enzyme 25-hydroxylase.
|
|
Synthesis and Metabolism
|
Cholecalciferol can be endogenously synthesized in the skin through exposure to ultraviolet B radiation or acquired from the diet or supplements. It undergoes a two-step activation process in the liver and kidneys to become biologically active as calcitriol.
|
|
Physiological Significance
|
Cholecalciferol, being the major form of vitamin D in nature, plays crucial roles in skeletal and extraskeletal functions. It is involved in various diseases due to its physiological actions.
|
|
Prevalence of Deficiency and Treatment
|
Vitamin D deficiency is significantly prevalent worldwide. Cholecalciferol, along with ergocalciferol and calcifediol, is used for the treatment of vitamin D insufficiency or deficiency.
|
|
Comparison with Ergocalciferol (Vitamin D2)
|
Cholecalciferol (D3) is distinguished from ergocalciferol (D2), with cholecalciferol being the form primarily found in animals. While both forms can be obtained through diet, cholecalciferol is considered the predominant form of vitamin D in nature.
|
|
Definition
|
A free vitamin D 3, isolated in crystalline state from the 3,5-dinitrobenzoate, produced by irradiation, and equivalent in activity to vitamin D3 of tunaliver oil.
|
|
General Description
|
Fine colorless crystals. Water insoluble.
|
|
Agricultural Uses
|
Rodenticide: Used in bait for vermin control. Vitamin D is a steroid hormone that has an important role in regulating body levels of calcium and phosphorus, and in mineralization of bone. Not approved for use in EU countries. Registered for use in the U.S. and other countries.
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego