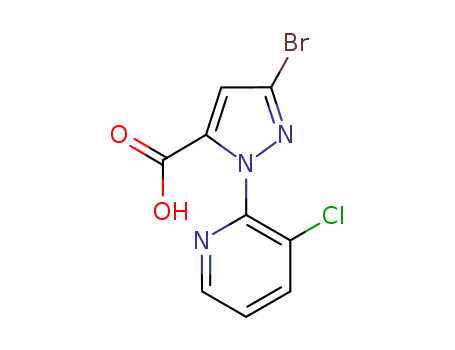
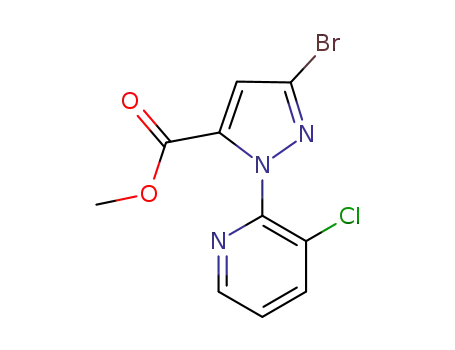
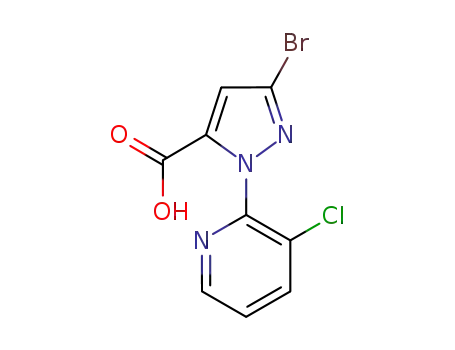
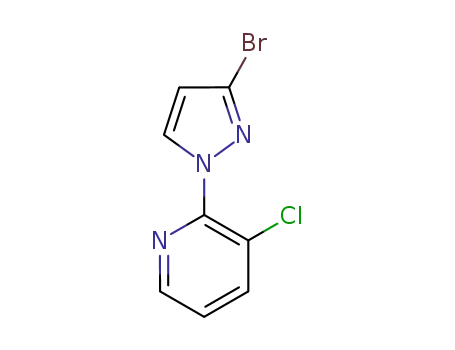
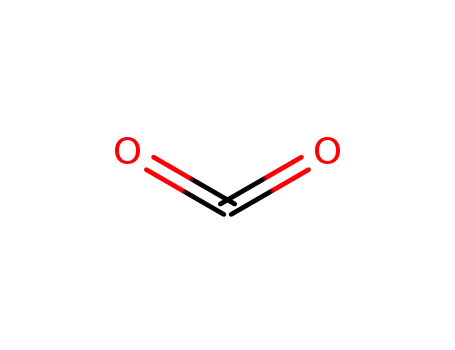
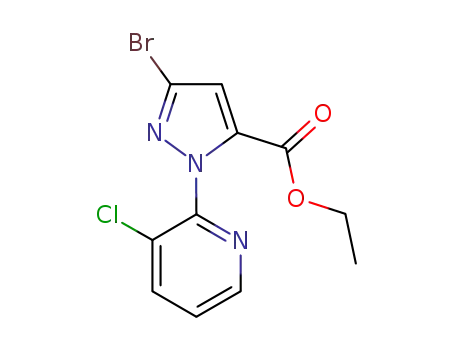
Factory Supply 500011-86-9 with Competitive Price, Buy Reliable Quality 3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid
- Molecular Formula:C9H5BrClN3O2
- Molecular Weight:302.515
- Vapor Pressure:0Pa at 20℃
- Melting Point:197-200 °C
- Boiling Point:477.4±45.0 °C(Predicted)
- PKA:2.39±0.36(Predicted)
- PSA:68.01000
- Density:1.92±0.1 g/cm3(Predicted)
- LogP:2.38140
500011-86-9 usage
3-Bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid is an organic compound that features a complex structure with multiple functional groups. It contains a pyrazole ring, a five-membered aromatic heterocycle with nitrogen atoms at the 1 and 2 positions, which is substituted with a bromine atom at the 3 position and a carboxylic acid group at the 5 position. Additionally, this pyrazole ring is linked to a 3-chloropyridine moiety, which is a six-membered aromatic ring with nitrogen at the 2 position and a chlorine atom at the 3 position. This compound likely has interesting chemical properties due to the presence of both electron-donating and electron-withdrawing groups, making it potentially useful in various chemical reactions and applications, particularly in the field of medicinal chemistry where such heterocyclic structures are common in bioactive molecules.
500011-86-9 Relevant articles
Design, synthesis and insecticidal-activity evaluation of N-pyridylpyrazolo-5-methyl amines and its derivatives
Yang, Shuai,Xu, Kaijie,Lai, Qiuqin,Zhao, Chen,Xu, Hanhong
, p. 4304 - 4311 (2020)
In searching for novel insecticidal lead...
PROCESS FOR PREPARATION OF SUBSTITUTED PYRAZOLES
-
Page/Page column 18-20, (2022/03/09)
: The present invention relates to the p...
METHODS FOR THE PREPARATION OF 5-BROMO-2-(3-CHLORO-PYRIDIN-2-YL)-2H-PYRAZOLE-3-CARBOXYLIC ACID
-
Paragraph 0256; 0257, (2021/04/23)
Described herein are novel methods of sy...
METHODS FOR THE PREPARATION OF 5-BROMO-2-(3-CHLORO-PYRIDIN-2-YL)-2H-PYRAZOLE-3-CARBOXYLIC ACID
-
Paragraph 0202; 0203, (2021/04/23)
Described herein are novel methods of sy...
METHODS FOR THE PREPARATION OF 5-BROMO-2-(3-CHLORO-PYRIDIN-2-YL)-2H-PYRAZOLE-3-CARBOXYLIC ACID
-
Paragraph 0299-0308, (2021/04/23)
Described herein are novel methods of sy...
500011-86-9 Process route
-
- 1045077-26-6
3-bromo-1-(3-chloropyridine-2-yl)-1H-pyrazole-5-carboxylic acid methyl ester
-
- 500011-86-9
3-bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylic acid
Conditions
| Conditions |
Yield |
|
With sodium hydroxide; In toluene; at 80 - 85 ℃; for 2h;
|
97% |
|
|
|
|
With sodium hydroxide; In water; at 80 ℃; for 2h; Inert atmosphere;
|
|
|
With methanol; sodium hydroxide; at 20 ℃; for 1h;
|
|
|
With sodium hydroxide; In toluene; at 80 - 85 ℃;
|
|
|
With sodium hydroxide; In toluene; at 80 - 85 ℃; for 2h;
|
|
|
With sodium hydroxide; In toluene; at 60 ℃; for 3h;
|
16.1 g |
-
- 500011-85-8
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine
-
- 124-38-9,18923-20-1
carbon dioxide
-
- 500011-86-9
3-bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylic acid
Conditions
| Conditions |
Yield |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With methylmagnesium bromide; diisopropylamine; In tetrahydrofuran; at 0 ℃; for 2.5h;
carbon dioxide; at 20 ℃; for 1h;
|
95% |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -78 ℃;
carbon dioxide; In tetrahydrofuran; Further stages.;
|
87% |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -78 ℃;
carbon dioxide; In tetrahydrofuran; at -78 - -20 ℃;
|
59% |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -76 - -71 ℃; for 0.25h;
carbon dioxide; In tetrahydrofuran; at -76 - -57 ℃; for 0.166667h;
|
|
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -76 - -71 ℃; for 0.25h;
carbon dioxide; In tetrahydrofuran; at -57 ℃; for 0.166667h;
With hydrogenchloride; In water;
|
|
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -76 ℃; for 0.25h;
carbon dioxide; In tetrahydrofuran; at -76 - -57 ℃; for 0.166667h;
|
|
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; n-heptane; ethylbenzene; at -78 ℃;
carbon dioxide; In tetrahydrofuran; n-heptane; ethylbenzene; at -78 - 20 ℃; for 1h;
With water; sodium hydroxide; In tetrahydrofuran; n-heptane; ethylbenzene; at 20 ℃; pH=10 - 12;
|
|
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -76 ℃; for 0.25h;
carbon dioxide; In tetrahydrofuran; at -76 - -57 ℃; for 0.166667h;
|
|
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at -76 - -71 ℃; for 0.25h;
carbon dioxide; In tetrahydrofuran; at -76 - -57 ℃; for 0.166667h;
|
27.7 g |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With lithium diisopropyl amide; In tetrahydrofuran; at 76 ℃; for 0.25h;
carbon dioxide; at 20 - 57 ℃;
|
27.7 g |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With methylmagnesium chloride; diisopropylamine; In tetrahydrofuran; at 0 ℃; for 2.5h;
carbon dioxide; In tetrahydrofuran; at 20 - 40 ℃;
|
30 g |
|
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine; With methylmagnesium chloride; diisopropylamine; lithium chloride; In tetrahydrofuran; toluene; at 5 - 15 ℃; for 6.75h;
carbon dioxide; In tetrahydrofuran; toluene; for 0.25h; Reagent/catalyst; Temperature; Solvent;
|
46 g |
500011-86-9 Upstream products
-
500011-85-8
2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine
-
124-38-9
carbon dioxide
-
500011-92-7
5-bromo-2-(3-chloro-pyridin-2-yl)-2H-pyrazole-3-carboxylic acid ethyl ester
-
1045077-26-6
3-bromo-1-(3-chloropyridine-2-yl)-1H-pyrazole-5-carboxylic acid methyl ester
500011-86-9 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego